
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
INFORMATION
Proxy Statement Pursuant to Section 14(a)
of the Securities Exchange Act of 1934
(Amendment No.__)
Filed by the Registrant |
|
☒ |
Filed by a Party other than the Registrant |
|
☐ |
Check the appropriate box:
☒ |
|
Preliminary Proxy Statement |
☐ |
|
Confidential, for Use of the Commission only (as permitted by Rule 14a-6(e)(2)) |
☐ |
|
Definitive Proxy Statement |
☐ |
|
Definitive Additional Materials |
☐ |
|
Soliciting Material Pursuant to § 240.14a-12 |
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box):
☒ |
No fee required. |
☐ |
Fee paid previously with preliminary materials. |
☐ |
Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11. |
222582143 v4
ATARA BIOTHERAPEUTICS, INC.
2380 Conejo Spectrum Street, Suite200
Thousand Oaks, CA 91320
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
To Be Held on May 31, 2023
Dear Stockholder:
You are cordially invited to attend the Annual Meeting of Stockholders of Atara Biotherapeutics, Inc., a Delaware corporation. The meeting will be held virtually via live audio webcast on May 31, 2023 at 9:00 a.m. Pacific time for the following purposes:
These items of business are more fully described in the Proxy Statement accompanying this Notice. The record date for the Annual Meeting is April 4, 2023 (the “Record Date”). Only stockholders of record at the close of business on that date may vote at the meeting or any adjournment thereof.
To increase access for all our stockholders, we have determined that it is prudent to hold this year’s annual meeting in a virtual-only format via live audio webcast. You may attend the virtual annual meeting at www.virtualshareholdermeeting.com/ATRA2023. To participate in the Annual Meeting, you will need the 16-digit control number that appears on your Notice of Internet Availability of Proxy Materials, proxy card or the instructions that accompanied your proxy materials. Refer to the “Questions and Answers About These Proxy Materials and Voting” section of the accompanying Proxy Statement for detailed procedures regarding attending, submitting questions and voting at the virtual Annual Meeting.
A list of stockholders entitled to vote at the meeting will be available for examination during normal business hours for ten days prior to the meeting for any purpose germane to the meeting at our corporate headquarters at 2380 Conejo Spectrum Street, Suite 200, Thousand Oaks, CA 91320. The stockholder list will also be available during the meeting at www.virtualshareholdermeeting.com/ATRA2023.
Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting of Stockholders to Be Held on May 31, 2023 at 9:00 a.m. Pacific time held virtually at www.virtualshareholdermeeting.com/ATRA2023. The Proxy Statement and Atara’s Annual Report on Form 10-K for the fiscal year 2022 are available electronically at www.proxyvote.com.
|
By Order of the Board of Directors
/s/ Pascal Touchon
Pascal Touchon, D.V.M.
President and Chief Executive Officer
Thousand Oaks, California
April [__], 2023
You are cordially invited to attend the Annual Meeting virtually via live audio webcast. Whether or not you expect to attend the meeting, please complete, date, sign and return the proxy card mailed to you, or vote over the telephone or the internet as instructed in these materials, as promptly as possible in order to ensure your representation at the meeting. Stockholders who attend the virtual Annual Meeting should follow instructions at www.virtualshareholdermeeting.com/ATRA2023 to vote online during the Annual Meeting. Please note, however, that if your shares are held of record by a broker, bank or other nominee and you wish to vote at the virtual Annual Meeting, you must follow the instructions from that record holder. |
TABLE OF CONTENTS

ATARA BIOTHERAPEUTICS, INC.
2380 Conejo Spectrum Street, Suite 200, Thousand Oaks, CA 91320
PROXY STATEMENT
FOR THE 2023 ANNUAL MEETING OF STOCKHOLDERS
QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING
Why did I receive a notice regarding the availability of proxy materials on the internet?
Pursuant to rules adopted by the Securities and Exchange Commission (the “SEC”), we have elected to provide access to our proxy materials over the internet. Accordingly, we have sent you a Notice of Internet Availability of Proxy Materials (the “Notice”) because our Board of Directors (the “Board”) is soliciting your proxy to vote at the 2023 Annual Meeting of Stockholders (the “Annual Meeting”), including any votes related to adjournments or postponements of the meeting. All stockholders will have the ability to access the proxy materials on the website referred to in the Notice or request to receive a printed set of the proxy materials. Instructions on how to access the proxy materials over the internet or to request a printed copy may be found in the Notice. In this Proxy Statement, “we”, “us”, “our”, “Company” and “Atara” refer to Atara Biotherapeutics, Inc.
We intend to mail the Notice on or about April 10, 2023 to all stockholders of record entitled to vote at the Annual Meeting.
How do I attend the Annual Meeting?
The meeting will be held virtually via live audio webcast on May 31, 2023 at 9:00 a.m. Pacific time. The meeting will only be conducted virtually via webcast and there will be no physical meeting location.
Attending the Annual Meeting as a Stockholder of Record: Shares Registered in Your Name
Atara’s stockholders of record as of April 4, 2023 (the “Record Date”) can attend the Annual Meeting by accessing the meeting center at www.virtualshareholdermeeting.com/ATRA2023 and entering the 16-digit control number on the proxy card or Notice previously received. The webcast of the Annual Meeting will be archived for one year after the date of the Annual Meeting at www.virtualshareholdermeeting.com/ATRA2023. Instructions on how to connect to the Annual Meeting and participate via the Internet, including how to demonstrate proof of stock ownership, are also available at the meeting website. If you do not have your 16-digit control number, you will be able to access and listen to the Annual Meeting, but you will not be able to vote your shares or submit questions during the Annual Meeting. See below, “Attending the Annual Meeting as a Guest.”
Attending the Annual Meeting as a Beneficial Owner: Shares Registered in the Name of a Broker, Bank or Other Nominee
Beneficial stockholders as of the Record Date (i.e., shares held in “street name” through an intermediary, such as a broker, bank or other nominee), who want to attend the Annual Meeting can attend using the 16-digit control number found on the Notice and instructions received from their broker, bank or other nominee.
Attending the Annual Meeting as a Guest
Guests may enter the Annual Meeting in “listen-only” mode by entering the Annual Meeting at www.virtualshareholdermeeting.com/ATRA2023 and entering the information requested in the “Guest Login” section. Guests will not have the ability to vote or ask questions during the Annual Meeting.
1
What if I have technical difficulties or trouble accessing the virtual Annual Meeting?
If you encounter any difficulties accessing the virtual meeting or during the meeting time, please call the phone number posted on the date of the meeting at www.virtualshareholdermeeting.com/ATRA2023 for general technical questions.
Who can vote at the Annual Meeting?
Only stockholders of record at the close of business on the Record Date will be entitled to vote at the Annual Meeting. On this Record Date, there were [x] shares of common stock outstanding and entitled to vote. Each outstanding share of common stock shall entitle the holder thereof to one vote.
Stockholder of Record: Shares Registered in Your Name
If on the Record Date your shares were registered directly in your name with our transfer agent, Computershare Trust Company, N.A. (“Computershare”), then you are a stockholder of record for purposes of the Annual Meeting. As a stockholder of record, you may vote virtually at the Annual Meeting or vote by proxy. Whether or not you plan to attend the virtual Annual Meeting, we urge you to fill out and return a proxy card or vote by proxy over the telephone or on the internet as instructed below to ensure your vote is counted.
Beneficial Owner: Shares Registered in the Name of a Broker, Bank or Other Nominee
If on the Record Date your shares were held, not in your name, but rather in an account at a brokerage firm, bank or other similar organization, then you are the beneficial owner of shares held in “street name” and the Notice is being forwarded to you by that organization. The broker, bank or other nominee holding your account is considered to be the stockholder of record for purposes of voting at the Annual Meeting. As a beneficial owner, you have the right to direct the broker, bank or other nominee holding your account regarding how to vote the shares in your account. You are also invited to attend the virtual Annual Meeting and may vote during the meeting if you log in with the 16-digit control number provided on your proxy materials.
Can I ask questions during the virtual Annual Meeting?
If you are attending the virtual Annual Meeting and logged in as a stockholder, questions can be submitted by accessing the meeting center at www.virtualshareholdermeeting.com/ATRA2023 and entering your 16-digit control number. Instructions on how to participate in the Annual Meeting are available on the meeting website.
What am I voting on?
There are four matters scheduled for a vote:
What if another matter is properly brought before the Annual Meeting?
We currently know of no other matters that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before the Annual Meeting, it is the intention of the proxyholders named in the accompanying proxy to vote on those matters in accordance with their best judgment.
2
How do I vote?
You may either vote “For” all the nominees to the Board or you may “Withhold” your vote for any nominee you specify. For each of the other matters to be voted on, you may either vote “For” or “Against” or abstain from voting.
The procedures for voting are as follows:
Stockholder of Record: Shares Registered in Your Name
If you are a stockholder of record, you may vote virtually during the Annual Meeting, vote by proxy over the telephone, vote by proxy through the internet or vote by proxy using a proxy card that you may request or that we may elect to deliver at a later time. Whether or not you plan to attend the Annual Meeting, we urge you to vote by proxy to ensure your vote is counted. You may still attend the Annual Meeting and vote during the virtual Annual Meeting even if you have already submitted a proxy.
Beneficial Owner: Shares Registered in the Name of Broker, Bank or Other Nominee
If you are a beneficial owner of shares registered in the name of your broker, bank or other nominee, you should receive a Notice containing voting instructions from that broker, bank or other nominee rather than from Atara. Simply follow the voting instructions in the Notice to ensure that your vote is counted. Follow the instructions from your broker, bank or other nominee included with the proxy materials, or contact your broker, bank or other nominee to request a proxy form. You are also invited to attend the virtual Annual Meeting and may vote during the meeting if you log in with the 16-digit control number provided on your proxy materials.
How many votes do I have?
On each matter to be voted upon, you have one vote for each share of common stock you owned as of April 4, 2023.
What happens if I do not vote?
Stockholder of Record: Shares Registered in Your Name
If you are a stockholder of record and do not vote by completing your proxy card, by telephone, through the internet or during the virtual Annual Meeting, your shares will not be voted.
Beneficial Owner: Shares Registered in the Name of Broker, Bank or Other Nominee
If you are a beneficial owner and do not instruct your broker, bank, or other nominee how to vote your shares, the question of whether your broker, bank or other nominee will still be able to vote your shares depends on whether the particular proposal is deemed to be a “routine” matter under the rules of various securities exchanges. Brokers, banks and nominees can use their discretion to vote “uninstructed” shares with respect to matters that are considered to be “routine,” but not with respect to “non-routine” matters. Under the applicable rules, “non-routine” matters are matters that may substantially affect the rights or privileges of stockholders, such as mergers, stockholder proposals, elections of directors (even if not contested), executive compensation (including any advisory stockholder votes on executive compensation and on the frequency of stockholder votes on executive compensation), and certain corporate governance proposals, even if supported by management. Accordingly, your
3
nominee may not vote your shares on Proposals 1, 2 and 4 without your instructions, but may vote your shares on Proposal 3, even in the absence of your instruction.
What if I return a proxy card or otherwise vote but do not make specific choices?
If you return a signed and dated proxy card or otherwise vote without marking voting selections, your shares will be voted, as applicable, “For” the election of each of the nominees for director; “For” the advisory approval of named executive officer compensation; and “For” ratification of selection by the Audit Committee of our Board of Deloitte & Touche LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2023. If any other matter is properly presented at the Annual Meeting, your proxyholder will vote your shares using their best judgment.
Who is paying for this proxy solicitation?
We will pay for the entire cost of soliciting proxies. Directors and employees will not be paid any additional compensation for soliciting proxies. We may also reimburse brokerage firms, banks, and other similar organizations for the cost of forwarding proxy materials to beneficial owners.
What does it mean if I receive more than one Notice?
If you receive more than one Notice, your shares may be registered in more than one name or in different accounts. Please follow the voting instructions on each of the Notices you receive to ensure that all of your shares are voted.
Can I change my vote after submitting my proxy?
Stockholder of Record: Shares Registered in Your Name
Yes. You can revoke your proxy at any time before the final vote at the Annual Meeting. If you are the record holder of your shares, you may revoke your proxy in any one of the following ways:
Simply attending the Annual Meeting will not, by itself, revoke your proxy. Your most current proxy card or telephone or internet proxy is the one that is counted.
Beneficial Owner: Shares Registered in the Name of Broker or Bank
If your shares are held by your broker, bank or other similar organization as a nominee or agent, you should follow the instructions provided by your broker, bank, or other similar organization.
4
When are stockholder proposals due for next year’s annual meeting?
To be considered for inclusion in next year’s proxy materials, your proposal must be submitted in writing by December [__], 2023 to our Secretary at 2380 Conejo Spectrum Street, Suite 200, Thousand Oaks, CA 91320, and must comply with all applicable requirements of Rule 14a-8 promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”); provided, however, that if our 2024 Annual Meeting of Stockholders is held before May 1, 2024 or after June 30, 2024, then the deadline is a reasonable amount of time prior to the date we print and mail the Notice for the 2024 Annual Meeting of Stockholders. If you wish to submit a proposal (including a director nomination) that is not to be included in next year’s proxy materials, the proposal must be received by our Secretary not later than the close of business on March 2, 2024 nor earlier than the close of business on February 1, 2024; provided, however, that if our 2024 Annual Meeting of Stockholders is held before May 1, 2024 or after June 30, 2024, then the proposal must be received no earlier than the close of business on the 120th day prior to such meeting and not later than the close of business on the later of the 90th day prior to such meeting or the 10th day following the day on which public announcement of the date of such meeting is first made. You are also advised to review our bylaws, which contain additional requirements about advance notice of stockholder proposals and director nominations.
In order to comply with the universal proxy rules, stockholders who intend to solicit proxies in support of director nominees other than our nominees must provide notice that sets forth the information required by Rule 14a-19 under the Exchange Act no later than April 1, 2024; provided, however, that if our 2024 Annual Meeting of Stockholders is held before May 1, 2024 or after June 30, 2024, then the notice must be received by the later of 60 days prior to the date of the annual meeting or the 10th day following the day on which public announcement of the date of such meeting is made.
What are “broker non-votes”?
As discussed above, when a beneficial owner of shares held in “street name” does not give instructions to the broker, bank or other nominee holding the shares as nominee as to how to vote on matters deemed to be “non-routine,” the nominee cannot vote the shares. These unvoted shares are counted as “broker non-votes.”
5
How many votes are needed to approve each proposal?
The following table summarizes the minimum vote needed to approve each proposal and the effect of abstentions and broker non-votes.
Proposal Number |
|
Proposal Description |
|
Vote Required for Approval |
|
Effect of Abstentions |
|
Effect of Broker Non-Votes |
1 |
|
Election of directors |
|
Plurality of the votes of shares present in person, present by remote communication, if applicable, or represented by proxy and entitled to vote on the matter
|
|
None |
|
None |
2 |
|
Advisory approval of the compensation of the Company’s named executive officers |
|
“For” votes from the holders of a majority of shares present in person, present by remote communication, if applicable, or represented by proxy and entitled to vote on the matter
|
|
Against |
|
None |
3 |
|
Ratification of the selection of Deloitte & Touche LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2023 |
|
“For” votes from the holders of a majority of shares present in person, present by remote communication, if applicable, or represented by proxy and entitled to vote on the matter |
|
Against |
|
Not Applicable(1) |
4 |
|
Approval of an amendment to the Company’s Certificate of Incorporation to provide for the exculpation of officers as permitted by Delaware law |
|
“For” votes from the holders of at least 66 2/3% of the voting power of all of the then-outstanding shares of capital stock of the Company entitled to vote generally in the election of directors |
|
Against |
|
None |
What is the quorum requirement?
A quorum of stockholders is necessary to hold a valid Annual Meeting. A quorum will be present if stockholders holding at least a majority of the outstanding shares entitled to vote are present at the Annual Meeting in person by remote communication or represented by proxy. On the Record Date, there were [x] shares outstanding and entitled to vote. Thus, the holders of [x] shares must be present in person by remote communication or represented by proxy at the Annual Meeting to have a quorum.
Your shares will be counted towards the quorum only if you submit a valid proxy (or one is submitted on your behalf by your nominee) or if you vote in person by remote communication at the Annual Meeting. Abstentions and broker non-votes will be counted towards the quorum requirement. If there is no quorum, the holders of a majority of shares present at the Annual Meeting in person by remote communication or represented by proxy may adjourn the Annual Meeting to another date.
How can I find out the results of the voting at the Annual Meeting?
Preliminary voting results will be announced at the Annual Meeting. In addition, final voting results will be published in a current report on Form 8-K that we expect to file within four business days after the Annual Meeting. If final voting results are not available to us in time to file a Form 8-K within four business days after the Annual Meeting, we intend to file a Form 8-K to publish preliminary results and, within four business days after the final results are known to us, file an additional Form 8-K to publish the final results.
6
PROPOSAL 1
ELECTION OF DIRECTORS
The Board is divided into three classes. Each class has a three-year term. Vacancies on the Board may be filled only by persons elected by a majority of the remaining directors. A director elected by the Board to fill a vacancy in a class, including vacancies created by an increase in the number of directors, shall serve for the remainder of the full term of that class and until the director’s successor is duly elected and qualified.
The Board presently has eight members. There are three directors in the class whose term of office expires in 2023. Drs. Gallagher and Touchon were previously elected to the Board by our stockholders. Dr. Roncarolo was appointed by the Board in 2020 and was identified by a third-party search firm for evaluation by the Nominating and Corporate Governance Committee. Each of the nominees listed below is currently a member of our Board who has been recommended for reelection by the Nominating and Corporate Governance Committee and nominated for reelection by the Board. If elected at the Annual Meeting, each of these nominees would serve until the 2026 Annual Meeting of Stockholders and until his or her successor has been duly elected and qualified, or, if sooner, until the director’s death, resignation or removal. It is our policy to invite and encourage directors and nominees for director to attend the Annual Meeting. All of our then-serving directors other than Drs. Seidenberg and Roncarolo and Mr. Mallik attended our 2022 Annual Meeting of Stockholders.
Directors are elected by a plurality of the votes of the holders of shares present in person, present by remote communication, if applicable, or represented by proxy and entitled to vote on the election of directors. This means that the three nominees receiving the highest number of affirmative votes, even if less than a majority of the shares outstanding on the record date, will be elected. Shares represented by executed proxies will be voted, if authority to do so is not withheld, for the election of the three nominees named below. If any nominee becomes unavailable for election as a result of an unexpected occurrence, shares that would have been voted for that nominee will instead be voted for the election of a substitute nominee proposed by the Board. Each person nominated for election has agreed to serve if elected. We have no reason to believe that any nominee will be unable to serve.
The following is a brief biography of each nominee and each director whose term will continue after the Annual Meeting.
Nominees for Election for a Three-year Term Expiring at the 2026 Annual Meeting
Pascal Touchon, D.V.M., 60, has served as our President and Chief Executive Officer and a member of the Board since June 2019. Prior to joining the Company, Dr. Touchon has served in roles of increasing responsibility at Novartis Oncology, a business unit of Novartis International AG, a global pharmaceutical company, since August 2015, most recently as Global Head Cell & Gene Therapies Oncology and a member of the Oncology Executive Committee. Previously, Dr. Touchon was Global Head Strategy, Business Development & Licensing, Oncology and a member of the Oncology Executive Committee. Prior to joining Novartis, Dr. Touchon spent nearly 30 years in the pharmaceutical industry in various companies, countries and leadership roles, including with Servier SAS, a privately owned French pharmaceutical company, as Senior Executive Vice President, member of the company Executive Committee and Head of Business Development and Licensing. Dr. Touchon currently serves as a director of a private biotechnology company and previously served on the boards of several other biotechnology companies. Dr. Touchon holds a Doctorate in Veterinary Medicine from Paul Sabatier University (Toulouse, France), a Diplôme d’Etudes Supérieures Spécialisées (DESS) in Management from Institut d’Administration des Entreprises (Toulouse, France) and an MBA from INSEAD (Fontainebleau, France). We believe that Dr. Touchon is qualified to serve on our Board due to his role as our President and Chief Executive Officer, his extensive experience in the pharmaceuticals industry and his leadership and management experience.
Carol Gallagher, Pharm.D., 58, has served as a member of the Board since January 2013 and as chair of the Board since December 2022. Dr. Gallagher is a seasoned public director, currently serving on two boards and seven previous boards. Starting in October 2014, Dr. Gallagher served as a partner with New Enterprise Associates, a venture capital firm, and she now serves as a venture advisor, a part-time role. She also serves as a Trustee on the Board of Trustees for the Salk Institute. Prior to joining New Enterprise Associates, Dr. Gallagher served as a venture partner with Frazier Healthcare, a venture capital firm, from October 2013 to September 2014. Dr. Gallagher served as the President and Chief Executive Officer of Calistoga Pharmaceuticals, a biopharmaceutical company, from 2008 to 2011, when the company was acquired by Gilead. From 2007 to 2008, Dr. Gallagher was the President and Chief Executive Officer of Metastatix, Inc., a biopharmaceutical company. Prior to that time starting in 1989, she served in various roles at pharmaceutical companies Eli Lilly and Company, Amgen, Agouron Pharmaceuticals, Pfizer, Biogen Inc., CancerVax, Inc. and Anadys Pharmaceuticals, Inc. Dr. Gallagher attended Vanderbilt University and holds B.S. and Doctor of Pharmacy degrees from the University of Kentucky. We believe that Dr. Gallagher is qualified to serve on our Board due to her extensive experience in the pharmaceuticals industry, her leadership and management experience, and her service as a director of other biopharmaceutical companies.
7
Maria Grazia Roncarolo, M.D., 68, has served as a member of the Board since May 2020. Dr. Roncarolo serves as the Professor of Pediatrics and Medicine at Stanford University, and Co-Director of the Stanford Institute for Stem Cell Biology and Regenerative Medicine, positions she has held since June 2014. From 2007 to 2014, Dr. Roncarolo served as Professor of Pediatrics at the School of Medicine and Surgery, San Raffaele Vita-Salute University, in Milan. From 2008 to 2013, Dr. Roncarolo served as the Scientific Director of the San Raffaele Scientific Institute in Milan. From 2000 to 2007, she served as the Director of the San Raffaele Telethon Institute for Gene Therapy in Milan. Prior to joining San Raffaele, she was a scientific staff member of the DNAX Research Institute of Molecular and Cellular Biology. In 2005, Dr. Roncarolo was elected member of the Academia Europea of Sciences. In 2000, she was awarded the honor of Ufficiale dell’Ordine “Al Merito della Repubblica Italiana” for scientific merits. Leading up to that, Dr. Roncarolo actively collaborated in the development of research programs for industry and biotechnology companies, including service as Co-Chair of the Scientific Advisory Board of GlaxoSmithKline plc for cell and gene therapy from 2016 to 2018, as Consultant for Novartis Pharmaceuticals in the areas of immunology, transplantation and gene transfer from 1997 to 2002, and as a founding member of the scientific advisory board for Kinetix Pharmaceuticals from 1997 to 2000. Dr. Roncarolo has served on the board of directors of Cosmo Pharmaceuticals NV since April 2012. She previously served on the board of directors of Graphite Bio, Inc. from 2019 until 2021. Dr. Roncarolo holds a medical degree from the University of Torino. We believe that Dr. Roncarolo is qualified to serve on our Board due to her deep industry knowledge and experience leading research efforts in the field.
THE BOARD OF DIRECTORS RECOMMENDS
A VOTE IN FAVOR OF EACH NAMED NOMINEE.
Directors Continuing in Office Until the 2024 Annual Meeting
Eric L. Dobmeier, 54, has served as a member of the Board since March 2015. Mr. Dobmeier has served as President and Chief Executive Officer, and as a member of the board of directors, of Chinook Therapeutics, Inc., a biotechnology company, since April 2019 and as a member of the board of directors of Structure Therapeutics, Inc. since January 2023. From January 2018 to June 2018, Mr. Dobmeier was President and Chief Executive Officer of Silverback Therapeutics, Inc., a biotechnology company. Prior to that, he was at Seattle Genetics, Inc., a biotechnology company, from 2002 to 2017, where he held positions of increasing responsibility, most recently as Chief Operating Officer from June 2011 to December 2017. Prior to joining Seattle Genetics, Mr. Dobmeier was an attorney with the law firms of Venture Law Group and Heller Ehrman LLP, where he represented technology companies in connection with public and private financings, mergers and acquisitions and corporate partnering transactions. Mr. Dobmeier formerly served on the board of directors of Stemline Therapeutics, Inc. from 2012 to 2018, Versartis, Inc. from 2017 to 2018 and Adaptive Biotechnologies Corporation from 2016 to 2021. Mr. Dobmeier received a J.D. from the University of California, Berkeley School of Law and an A.B. in History from Princeton University. We believe that Mr. Dobmeier is qualified to serve on our Board due to his legal, business development, and operating experience, years of senior management experience at a public biotechnology company, and his service as a director of other biopharmaceutical companies.
William K. Heiden, 63, has served as a member of the Board since November 2015. Mr. Heiden served as the President and Chief Executive Officer, and as a member of the board of directors, of AMAG Pharmaceuticals, Inc., a pharmaceutical company, from May 2012 until April 2020. Prior to joining AMAG, Mr. Heiden served as President and Chief Executive Officer of GTC Biotherapeutics, Inc. (now part of LFB, S.A.), a biotherapeutics company, from June 2010 to May 2012. From September 2004 until December 2008, Mr. Heiden served as President and Chief Executive Officer of Elixir Pharmaceuticals, Inc., a biopharmaceutical company. Prior to joining Elixir Pharmaceuticals, Mr. Heiden served as President and Chief Operating Officer of Praecis Pharmaceuticals Incorporated (which was acquired by GlaxoSmithKline), from 2002 to 2004. From 1987 to 2002, Mr. Heiden progressed through various positions of increasing responsibility at Schering-Plough Corporation (which was acquired by Merck & Co.), including managing a number of businesses in the United States, Europe and Canada. Mr. Heiden has served on the board of directors of Macrogenics, Inc. since May 2022. Mr. Heiden holds an M.B.A. from Cornell University’s Johnson Graduate School of Management, a M.I.M. degree from the University of Louvain, and a B.A. degree from the University of Florida. We believe that Mr. Heiden is qualified to serve on our Board due to his extensive experience as a pharmaceutical and biotechnology executive.
Ameet Mallik, 50, has served as a member of the Board since August 2021. Mr. Mallik currently serves as President and Chief Executive Officer as well as a member of the board of directors, of ADC Therapeutics since May 2022. Prior to this, Mr. Mallik served as the Chief Executive Officer of Rafael Holdings, Inc. (“Rafael”), a biopharmaceutical company, from May 2021 until March 2022. Prior to joining Rafael, Mr. Mallik served in roles of increasing responsibility at Novartis International AG (“Novartis”), a global pharmaceutical company. From November 2017 to May 2021, Mr. Mallik served as Executive Vice President and Head, U.S. Oncology of Novartis. In this role, Mr. Mallik was responsible for Novartis’s commercial and medical oncology operations in the United States. From November 2015 to November 2017, Mr. Mallik served as Global Head,
8
Marketing, Value and Access, and from April 2014 to November 2015 as Head, Latin America and Canada, both for Novartis Oncology. Mr. Mallik began his career at Novartis as Head of Strategic Planning and held a number of commercial leadership roles at Novartis and Sandoz. At Sandoz, Mr. Mallik was Head of Biopharmaceuticals & Oncology Injectables. Mr. Mallik previously worked as an Associate Principal at McKinsey and Company. Mr. Mallik serves a director on the board of Rafael Holdings, Inc., a position he has held since March 2022. Mr. Mallik previously served on the Health Section Governing Board of BIO, the world’s largest trade association representing biotechnology companies and institutions. Mr. Mallik holds a B.S. in Chemical Engineering and an M.S. in Biotechnology from Northwestern University and an M.B.A. from The Wharton School at the University of Pennsylvania. We believe that Mr. Mallik is qualified to serve on our Board due to his extensive experience as a pharmaceutical and biotechnology executive, particularly in oncology and with regard to commercial strategy and market access.
Beth Seidenberg, M.D., 65, has served as a member of the Board since our founding in August 2012. Dr. Seidenberg is the managing director of Westlake Village BioPartners, a venture capital firm that focused on life sciences that she founded in September 2018. Dr. Seidenberg is also a General Partner at Kleiner Perkins Caufield & Byers, a venture capital firm, where she has primarily focused on life sciences investing since May 2005. Dr. Seidenberg was previously the Senior Vice President, Head of Global Development and Chief Medical Officer at Amgen, Inc., a biotechnology company. In addition, Dr. Seidenberg was a senior executive in research and development at Bristol Myers Squibb Company, a biopharmaceutical company, and Merck. Dr. Seidenberg received a B.S. from Barnard College and an M.D. from the University of Miami School of Medicine and completed her post-graduate training at The Johns Hopkins University, George Washington University, and the National Institutes of Health. Dr. Seidenberg has served on the board of directors of Progyny, Inc., since 2010, and as a member of the board of directors of Vera Therapeutics, Inc., since 2016. Dr. Seidenberg formerly served on the board of directors of TESARO from 2011 to 2018, ARMO BioScience from 2012 to 2018, and Epizyme, Inc. from 2008 to 2019. We believe that Dr. Seidenberg is qualified to serve on our Board due to her extensive experience in the life sciences industry as a senior executive and venture capitalist, as well as her training as a physician.
Directors Continuing in Office Until the 2025 Annual Meeting
Matthew K. Fust, 58, has served as a member of the Board since March 2014. Mr. Fust is a board member and advisor to life sciences companies. Mr. Fust has served on the board of directors of Crinetics Pharmaceuticals, Inc. since February 2018, and Ultragenyx Pharmaceutical, Inc. since January 2014. Mr. Fust formerly served of the board of directors of Sunesis Pharmaceuticals, Inc. from 2005 to 2017, MacroGenics, Inc. from March 2014 until the expiration of his term in May 2020, and Dermira, Inc. from April 2014 through its acquisition by Eli Lilly and Company in February 2020. Mr. Fust was previously Executive Vice President and Chief Financial Officer of Onyx Pharmaceuticals, Inc., a biopharmaceutical company, from January 2009 through its acquisition by Amgen in October 2013. Mr. Fust continued as an employee of Amgen until January 2014. From May 2003 to December 2008, Mr. Fust served as Chief Financial Officer at Jazz Pharmaceuticals, Inc., a specialty pharmaceutical company. From 2002 to 2003, Mr. Fust served as Chief Financial Officer at Perlegen Sciences, a biopharmaceutical company. Previously, he was Senior Vice President and Chief Financial Officer at ALZA Corporation, a pharmaceutical company, where he was an executive from 1996 until 2002. From 1991 until 1996, Mr. Fust was a manager in the healthcare strategy practice at Andersen Consulting. Mr. Fust holds a B.A. from the University of Minnesota and an M.B.A. from the Stanford Graduate School of Business. We believe that Mr. Fust is qualified to serve on our Board due to his extensive experience as a chief financial officer in the life sciences industry, his leadership and management experience, and his service as a director of other biopharmaceutical companies.
9
INFORMATION REGARDING THE BOARD OF DIRECTORS AND CORPORATE GOVERNANCE
Independence of the Board of Directors
Generally, under the listing requirements and rules of The Nasdaq Stock Market (“Nasdaq”), independent directors must comprise a majority of a listed company’s board of directors. The Board has undertaken a review of its composition, the composition of its committees and the independence of each director. The Board has determined that, other than Dr. Touchon, by virtue of his position as President and Chief Executive Officer, none of our directors has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each is “independent” as that term is defined under the listing requirements and rules of Nasdaq. Accordingly, a majority of the members of the Board is independent, as required under applicable Nasdaq rules. In making this determination, the Board considered the current and prior relationships that each non-employee director has with the Company and all other facts and circumstances the Board deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
Board Leadership Structure
Dr. Gallagher, an independent director, currently serves as the chair of the Board. As chair of the Board, Dr. Gallagher establishes the agenda for regular Board meetings, presides over Board meetings, presides over periodic meetings of the Board’s independent directors, serves as a liaison between our Chief Executive Officer and the independent directors and performs such additional duties as the Board may otherwise determine and delegate. Accordingly, the chair of the Board has substantial ability to shape the work of the Board. We believe that separation of the positions of the chair of the Board and Chief Executive Officer reinforces the independence of the Board in its oversight of the business and affairs of the Company. In addition, we believe that having an independent chair of the Board creates an environment that is more conducive to objective evaluation and oversight of management’s performance, increasing management accountability and improving the ability of the Board to monitor whether management’s actions are in the best interests of the Company and its stockholders. As a result, we believe having an independent chair of the Board can enhance the effectiveness of the Board as a whole.
Role of the Board in Risk Oversight
One of the Board’s key functions is informed oversight of our risk management process. The Board does not have a standing risk management committee, but rather administers this oversight function directly through the Board as a whole, as well as through various Board standing committees that address risks inherent in their respective areas of oversight. In particular, the Board is responsible for monitoring and assessing strategic risk exposure, including a determination of the nature and level of risk appropriate for the Company. The Audit Committee has the responsibility to consider and discuss our major financial and cybersecurity risk exposures, insurance coverage, and the steps management has taken to monitor and control these exposures, including guidelines and policies designed to mitigate risks identified. The Audit Committee also monitors compliance with legal and regulatory requirements, in addition to oversight of the performance of our internal audit function. The Nominating and Corporate Governance Committee monitors the effectiveness of our corporate governance guidelines, including whether they are successful in preventing illegal or improper liability-creating conduct. The Nominating and Corporate Governance Committee also has oversight of the Company’s practices with respect to enterprise risk assessment and risk management, while management is responsible for day-to-day risk management processes. The Compensation Committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking. It is the responsibility of the committee chairs to report findings regarding material risk exposures to the Board as quickly as possible. The Board has delegated to the chair of the Board the responsibility of coordinating between the Board and management with regard to the determination and implementation of responses to any problematic risk management issues.
10
Meetings of the Board of Directors
The Board met six times during 2022. Each Board member attended 75% or more of the aggregate number of meetings of the Board and of the committees on which they served held during the portion of the last fiscal year for which they were a director or committee member. In addition, in 2022, our non-employee directors met four times in regularly scheduled executive sessions at which only non-employee directors were present. Mr. Renaud served as chair for Board meetings and presided over the executive sessions until his resignation in December 2022.
Information Regarding Committees of the Board of Directors
The Board has four standing committees: an Audit Committee, a Compensation Committee, a Nominating and Corporate Governance Committee, and a Research and Development Committee. The following table provides membership and meeting information for each of the Board committees in 2022:
Name |
|
Audit |
|
Compensation |
|
Nominating and Corporate Governance |
Research and Development |
Pascal Touchon, D.V.M. |
|
|
|
|
|
|
X* |
Roy Baynes, M.D., Ph.D. (1) |
|
|
|
X(1) |
|
|
X(1) |
Eric L. Dobmeier |
|
X |
|
X* |
|
|
|
Matthew K. Fust |
|
X* |
|
|
|
X(2) |
|
Carol Gallagher, Pharm.D.(3) |
|
|
|
X(3) |
|
X(3) |
|
William K. Heiden |
|
X |
|
X(4) |
|
|
|
Beth Seidenberg, M.D. |
|
|
|
|
|
X* |
X |
Ronald C. Renaud, Jr. (5) |
|
|
|
|
|
|
|
Maria Grazia Roncarolo, M.D. (6) |
|
|
|
|
|
X(6) |
X |
Ameet Mallik (7) |
|
|
|
X(7) |
|
X(7) |
|
|
|
|
|
|
|
|
|
Total meetings in 2022 |
|
6 |
|
6 |
|
2 |
5 |
* Committee Chair.
Audit Committee
The Board has determined that each member of the Audit Committee is independent under Nasdaq listing standards and Rule 10A-3(b)(1) promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The Board has also determined that Mr. Fust is an “audit committee financial expert” within the meaning of SEC regulations. Each member of the Audit Committee has the requisite financial expertise required under the applicable requirements of Nasdaq. In arriving at this determination, the Board examined each Audit Committee member’s scope of experience and the nature of their employment in the corporate finance sector. The primary functions of this committee include, among others:
11
The Audit Committee is briefed regularly on cybersecurity matters, such as our cybersecurity defense measures, current trends and cybersecurity incidents, including our responses to such incidents, if appropriate, and updates to our continuous and comprehensive employee cybersecurity training programs.
The Audit Committee has authority to engage legal counsel or other experts or consultants, as it deems appropriate to carry out its responsibilities. The Board has adopted a written Audit Committee charter that is available to stockholders on our website at http://investors.atarabio.com/governance.
Report of the Audit Committee of the Board of Directors1
The Audit Committee has reviewed and discussed the audited financial statements for the fiscal year ended December 31, 2022 with our management. The Audit Committee has discussed with the independent registered public accounting firm the matters required to be discussed by the applicable requirements of the Public Company Accounting Oversight Board (“PCAOB”) and the SEC. The Audit Committee has also received the written disclosures and the letter from the independent registered public accounting firm required by applicable requirements of the PCAOB regarding the independent accountants’ communications with the Audit Committee concerning independence and has discussed with the independent registered public accounting firm the accounting firm’s independence. Based on the foregoing, the Audit Committee has recommended to the Board that the audited financial statements be included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022.
Matthew K. Fust
Eric L. Dobmeier
William Heiden
Compensation Committee
The Board has determined that each member of the Compensation Committee is independent under Nasdaq listing standards and Rule 10c-1 promulgated under the Exchange Act, and a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act. The primary functions of this committee include, among others:
12
The Compensation Committee has authority to engage legal counsel or other experts or consultants, as it deems appropriate to carry out its responsibilities. The Board has adopted a written Compensation Committee charter that is available to stockholders on our website at http://investors.atarabio.com/governance.
Compensation Committee Processes and Procedures
The Compensation Committee met six times during 2022. The agenda for each meeting is usually developed by our Chief Executive Officer, Chief People Officer (from 2022 through February 2023), Chief Financial Officer, and Chief Legal Officer in consultation with the Chair of the Compensation Committee and our outside compensation consultants, if applicable. The Compensation Committee meets regularly in executive session. However, from time to time, various members of management and other employees, as well as outside advisors or consultants, may be invited by the Compensation Committee to make presentations, to provide financial or other background information or advice or to otherwise participate in Compensation Committee meetings. The Chief Executive Officer does not and will not participate in, or be present during, any deliberations or determinations of the Compensation Committee regarding their compensation. The charter of the Compensation Committee grants the Compensation Committee full access to all of our books, records, facilities and personnel. In addition, under the charter, the Compensation Committee has authority to engage legal counsel or other experts or consultants, as it deems appropriate to carry out its responsibilities. The Compensation Committee has the authority to retain compensation consultants to assist in its evaluation of executive and director compensation, including the authority to approve the consultant’s reasonable fees and other retention terms. Under the charter, the Compensation Committee may select, or receive advice from, a compensation consultant, legal counsel or other advisor to the Compensation Committee (other than in-house legal counsel and certain other types of advisors), only after assessing their independence in accordance with, and to the extent required by, applicable law and the listing requirements of Nasdaq; however, there is no requirement that any advisor be independent.
During the first half of 2022, the Compensation Committee engaged the Radford advisory team of the Rewards Solution practice at Aon (“Radford”) as independent compensation consultant, and transitioned to Pearl Meyer & Partners, LLC (“Pearl Meyer”) as its independent compensation consultant effective June 2022. After considering all of the factors required by applicable Nasdaq rules, the Compensation Committee was satisfied with Radford’s and Pearl Meyer’s independence and requested that its independent compensation consultants evaluate and help us refine our employee and non-employee director compensation strategies and practices. As part of its engagement, Pearl Meyer was requested by the Compensation Committee to develop a comparative group of companies and to perform analyses of competitive performance and compensation levels for that group. With respect to the compensation of the Chief Executive Officer, Pearl Meyer developed recommendations that were presented to the Compensation Committee for its consideration. Following a dialogue with Pearl Meyer, the Compensation Committee considered the recommendations in addition to corporate performance and approved the
13
recommendations subject to certain modifications deemed appropriate by the Compensation Committee. With respect to the executive officers, the Chief Executive Officer in consultation with Pearl Meyer developed recommendations that were presented to the Compensation Committee for its consideration. Following discussion with the Chief Executive Officer and with Pearl Meyer, the Compensation Committee considered the recommendations in addition to corporate and individual performance and approved the recommendations subject to certain modifications deemed appropriate by the Compensation Committee.
Historically, the Compensation Committee has made most of the significant adjustments to annual compensation and determined bonus and equity awards at one or more meetings held during the first quarter of the year. The Compensation Committee also considers matters related to individual compensation, such as compensation for new executive hires, as well as high-level strategic issues, such as the efficacy of our compensation strategy, potential modifications to that strategy, risks created by that strategy and new trends, retention concerns and plans or approaches to compensation, at various meetings throughout the year. For executives other than the Chief Executive Officer, the Compensation Committee solicits and considers evaluations and recommendations submitted to the Committee by the Chief Executive Officer. In the case of the Chief Executive Officer, the evaluation of his performance is conducted by the Compensation Committee with feedback from the Board as well as the executives, which determines any adjustments to Chief Executive Officer’s compensation as well as awards to be granted. For all executives and directors as part of its deliberations, the Compensation Committee may review and consider, as appropriate, materials that it deems relevant. These materials may include financial reports and projections, operational data, tax and accounting information, tally sheets that set forth the total compensation that may become payable to executives in various hypothetical scenarios, executive and director stock ownership information, company stock performance data, analyses of historical executive compensation levels and current compensation levels across our Company and recommendations of the Compensation Committee’s compensation consultant, including analyses of executive and director compensation paid at the comparative group of companies (with such group of companies developed by Pearl Meyer and agreed upon by the Compensation Committee).
Compensation Committee Interlocks and Insider Participation
None of the members of the Compensation Committee is currently or has been at any time one of our officers or employees. None of our executive officers currently serves, or has served during the last year, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of the Board or the Compensation Committee.
Nominating and Corporate Governance Committee
The Board has determined that each member of the Nominating and Corporate Governance Committee is independent under the Nasdaq listing standards. The primary functions of this committee include:
The Nominating and Corporate Governance Committee has authority to engage advisors or consultants (including legal counsel and search firms), as it deems appropriate to carry out its responsibilities. The Board has adopted a written Nominating and Corporate Governance Committee charter that is available to stockholders on our website at http://investors.atarabio.com/governance.
14
The Nominating and Corporate Governance Committee believes that candidates for director should have certain minimum qualifications, including the ability to read and understand basic financial statements, being over 21 years of age and having the highest personal integrity and ethics. In evaluating director nominee candidates, the Nominating and Corporate Governance Committee typically also considers factors such as: possessing relevant expertise upon which to be able to offer advice and guidance to management, having sufficient time to devote to our affairs, demonstrated excellence in their field, having the ability to exercise sound business judgment and having the commitment to rigorously represent the long-term interests of our stockholders and other factors deemed appropriate given then-current needs of the Board and Atara, to maintain a balance of knowledge, experience and capability. However, the Nominating and Corporate Governance Committee retains the right to modify the above qualifications from time to time.
The Board believes that diversity of viewpoints, background, experience, and other characteristics, such as race, gender, ethnicity, sexual orientation, culture and nationality, are an important part of its makeup, and the Nominating and Corporate Governance Committee and the Board actively seek these characteristics in identifying director candidates.
Candidates for director nominees are reviewed in the context of the current composition of the Board, our operating requirements, Nasdaq listing standards, applicable law and regulations and the long-term interests of stockholders. The Nominating and Corporate Governance Committee also determines whether a nominee is independent for Nasdaq purposes based upon Nasdaq listing standards, SEC rules and regulations and the advice of counsel, if necessary. The Nominating and Corporate Governance Committee conducts any appropriate and necessary inquiries into the backgrounds and qualifications of potential director candidates. The Nominating and Corporate Governance Committee meets to discuss and consider the candidates’ qualifications and ultimately recommend director nominees to the Board.
In the case of incumbent directors whose terms of office are set to expire, the Nominating and Corporate Governance Committee reviews these directors’ overall service to Atara during their terms, including the number of meetings attended, level of participation, quality of performance and any other relationships and transactions that might impair the directors’ independence.
The Nominating and Corporate Governance Committee will consider director candidates recommended by stockholders. The Nominating and Corporate Governance Committee does not intend to alter the manner in which it evaluates candidates, including the minimum criteria set forth above, based on whether or not the candidate was recommended by a stockholder. Stockholders who wish to recommend individuals for consideration by the Nominating and Corporate Governance Committee to become nominees for election to the Board may do so by delivering a written recommendation to the Nominating and Corporate Governance Committee at the following address: 2380 Conejo Spectrum Street, Suite 200, Thousand Oaks, CA 91320 not less than six months prior to any meeting at which directors are to be elected. Submissions must include the full name of the proposed nominee, a description of the proposed nominee’s business experience for at least the previous five years, complete biographical information, a description of the proposed nominee’s qualifications as a director and a representation that the nominating stockholder is a beneficial or record holder of our common stock and has been a holder for at least one year. Any such submission must be accompanied by the written consent of the proposed nominee to be named as a nominee and to serve as a director if elected.
Research and Development Committee
The Research and Development Committee provides advice and support to the Company in relation to the Company’s research and development activities and strategy. The primary function of this committee is to confer with the Chief Executive Officer and the Company’s research and development leadership team regarding:
15
The Board has adopted a written Research and Development Committee charter that is available to stockholders on our website at http://investors.atarabio.com/governance.
Ad Hoc Transactions Committee
The ad hoc Transactions Committee meets as needed to review and advise the Company in relation to key corporate transactions, including potential collaborations and licensing arrangements. The primary function of this ad hoc committee is to confer on such matters with the Chief Executive Officer and the Company’s corporate development, finance and legal leadership teams. The members of the ad hoc Transactions Committee are Drs. Gallagher and Touchon, and Messrs. Dobmeier and Mallik, each of whom have significant expertise in structuring, evaluating and advising on biotechnology collaboration and licensing transactions.
Summary of Current Director Core Experiences and Skills
Our Board members are a diverse group of highly qualified leaders in their respective fields. Most of our directors have senior leadership experience at large public and private companies and have significant and diverse management experience (including strategic, planning, financial reporting, compliance, risk management, leadership development, scientific and healthcare industry expertise). The Board believes the skills, qualities, attributes, experience and diversity of our directors provide us with a range of perspectives to effectively represent the best interests of our stockholders. The chart below summarizes our directors’ strengths.
Experience / Skills* |
|
|
|
|
|
|
|
|
Healthcare Industry, Providers and Payers |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
|
✓ |
Research and Development |
✓ |
|
|
✓ |
|
|
✓ |
✓ |
Regulatory / Compliance |
✓ |
✓ |
✓ |
✓ |
|
✓ |
✓ |
✓ |
Public Company Governance |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
Financial / Accounting |
|
✓ |
✓ |
|
✓ |
|
|
|
Executive Leadership |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
M&A / Transactions |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
|
✓ |
*The lack of a mark for a particular item does not mean the director does not possess the specific qualification, characteristic, skill or experience. Each of our directors has experience and/or skills in the enumerated areas, the mark is intended to indicate the director has a particular strength in such area.
Board Diversity and Tenure
Our Board is comprised of several individuals who self-identify as women or are from underrepresented communities. Our Board is committed to continuously align the composition of the Board and its leadership structure with our long-term strategic needs. The Nominating and Corporate Governance Committee identifies, evaluates, and makes recommendations to the Board. The Nominating and Corporate Governance Committee considers current Board skills, background, diversity, independence, experience, tenure, and anticipated retirements to identify gaps that may need to be filled on the Board. We recognize there is always more that can be done to promote diversity and inclusion and we actively seek ways to embrace diversity, inclusion, and equitable representation at all levels of our organization – and Atara is a much richer place for it.
16
Board Diversity Matrix
Total Number of Directors |
8 |
|
Part I: Gender Identity |
Female |
Male |
Directors |
3 |
5 |
Part II: Demographic Background |
|
|
Asian |
0 |
1 |
White |
3 |
4 |
|
|
|
LGBTQ+ |
0 |
1 |
Board Diversity Highlights |
Board Tenure |
|
|
|
|
Stockholder Communications with the Board
The Board has adopted a formal process by which stockholders may communicate with the Board or any of its directors. This information is available on our website at http://investors.atarabio.com/governance. Any interested person may also communicate directly with the chair of the Board or the independent or non-employee directors. Persons interested in communicating directly with the independent or non-employee directors regarding their concerns or issues are referred to the procedures for such communications on our website at http://investors.atarabio.com/governance/contact-board.
Code of Conduct
The Board has adopted a code of conduct that applies to all of our corporate employees, officers and directors, including those officers and employees responsible for financial reporting. Our code of conduct is available on our website at https://investors.atarabio.com/governance/governance-documents. We intend to disclose any amendments to this policy, or any waivers of its requirements, on our website or in public filings to the extent required by applicable SEC rules or exchange requirements.
Corporate Governance Guidelines
The Board has adopted Corporate Governance Guidelines to assure that the Board will have the necessary authority and practices in place to review and evaluate our business operations as needed and to make decisions that are independent of our management. The guidelines are also intended to align the interests of directors and management with those of our stockholders. The Corporate Governance Guidelines set forth the practices the Board intends to follow with respect to board composition and selection, board meetings and involvement of senior management, Chief Executive Officer performance evaluation and succession planning, and board committees and compensation. The Corporate Governance Guidelines also formalize the Board’s belief that a diversity of viewpoints, background, experience and other characteristics, such as race, gender, ethnicity, sexual orientation, culture and nationality, are an important part of its makeup, and that it actively seeks these characteristics in identifying director candidates.
17
Our Corporate Governance Guidelines are available on our website at https://investors.atarabio.com/governance/governance-documents.
Compliance Program
Our Compliance Program is designed to promote ethical business conduct and compliance with applicable laws and regulations. Key components of our compliance program include policies and procedures, compliance training and educational opportunities, maintaining avenues for staff to raise concerns without fear of retaliation, including anonymously through a business conduct hotline, and responding appropriately to compliance-related events.
Stock Ownership Guidelines
Our Stock Ownership Guidelines for our directors and named executive officers further align their financial interests with those of our stockholders, as well as promote sound corporate governance. For a detailed description of our Stock Ownership Guidelines see “Other Compensation Policies and Guidelines – Stock Ownership Guidelines” below.
Insider Trading Policy (including Anti-Hedging and Anti-Pledging)
Our Insider Trading Policy prohibits all employees (including executive officers) and directors from engaging in short sales, transactions in put or call options, hedging transactions or similar inherently speculative transactions with respect to our stock at any time. For a detailed description of our Insider Trading Policy see “Other Compensation Policies and Guidelines – Insider Trading Policy (including Anti-Hedging and Anti-Pledging)” below.
Clawback Policy
For a detailed description of our Clawback Policy see “Other Compensation Policies and Guidelines – Clawback Policy” below.
Environmental, Social, and Governance
Our mission is to transform the lives of patients with serious diseases through pioneering science, teamwork and a commitment to excellence.
We are committed to building a safe, environmentally sustainable and ethical business that provides long-term value for all Atara stakeholders.
As part of this commitment, we support Environmental, Social and Governance (“ESG”) initiatives aligned to our mission, culture and core values. These values provide the foundation for us to demonstrate our dedication to patients, staff, our environment and local communities. Our Board oversees the ESG initiatives and associated risks relevant to the Company.
Governance
We are committed to good corporate governance and conducting our business in an ethical manner. We have adopted numerous policies and guidelines to facilitate legal and ethical conduct and to further align the interests of our employees and directors with our stockholders and other key stakeholders, including the patients we serve. For a detailed description of several of these policies and guidelines, see “Information Regarding the Board of Directors and Corporate Governance” above.
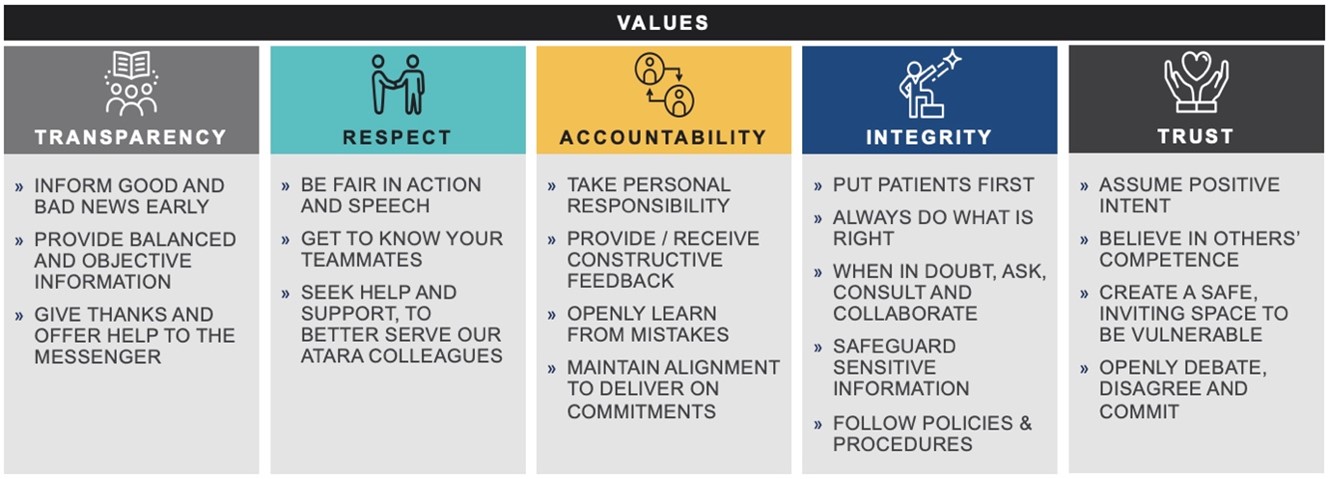
Transparency, Respect, Accountability, Integrity, and Trust (“TRAIT”) Values
We have formalized a set of company values to guide us in how we conduct our business, interact with colleagues and execute our corporate strategy.
18
Environmental Sustainability
We are committed to operating our facilities in an environmentally responsible way to reduce environmental impacts and protect our people, our business, the environment and the communities where we operate. In light of the potential impact our business may have on the environment, we have set goals and adopted a number of internal policies and management systems designed to eliminate, reduce, or substitute hazardous materials and waste and reduce water and energy consumption. For example, we constructed our Atara Research Center in Thousand Oaks, California with provisions made to comply with Title 24 requirements, including exterior wall insulation, LED lighting with energy reducing controls system, low flow restroom fixtures, drought tolerant landscaping design, waste stream segregation for landfill and recyclables, and elimination of chlorofluorocarbons and other harmful chemicals during construction.
Community
We are committed to fostering the growth of the next generation of leaders through our involvement in our communities. Through active engagement with local schools, we discuss careers in Science, Technology, Engineering, & Mathematics.
In addition, we have worked with local universities to provide summer internship opportunities at both the graduate and undergraduate levels. Additionally, for the National Day of Giving we partnered with James Storehouse, a local non-profit focused on serving kids in foster care to provide housing starter kits for youth aging out of the foster care system and setting up their first home.
In support of the National Multiple Sclerosis Society and the nearly 1 million people living with multiple sclerosis in the United States, each year we actively participate in local “Walk MS” events to raise funds to advance groundbreaking research and provide vital support and resources for the multiple sclerosis community.
We also collaborate with other biotech companies and our local community through the Alliance for Regenerative Medicine, Biocom California, the Healthcare Businesswoman’s Association, and the Conejo Valley Chamber of Commerce, amongst others. We look forward to continuing our community involvement to advance innovation and diversity in our industry.
Patients
We are committed to the highest standards of product quality and patient safety. Through the training of full-time staff, contract workers and vendors on our policies, standard operating procedures, work instructions and guidelines, we strive to carry out all activities to the standards and expectations for compliance and quality performance. Close oversight of the operations of our contracted manufacturing service providers makes certain their activities also adhere to our standards. Information on our current clinical studies can be found on our website at www.atarabio.com.
As the most advanced allogeneic T-cell immunotherapy company with the first ever regulatory approval in the world for an allogeneic T-cell immunotherapy, we intend to rapidly deliver off-the-shelf treatments to patients with high unmet medical need and continue to develop novel investigational treatments for patients with cancer and autoimmune diseases. We strive to make every effort to support patients in need of treatment options. We will consider providing an individual patient with access
19
to investigational product candidates through an expanded access study. More information on our Expanded Access Program and process for applying can be found on our website at https://www.atarabio.com/expanded-access-patients/.
Staff Engagement
We are dedicated to providing an inclusive, safe, and collaborative environment for all staff. Staff engagement and feedback is an integral part of helping us create and maintain an inclusive, safe and collaborative environment for our staff. We gather feedback periodically through multi-channel opportunities, such as climate surveys, company-wide surveys, culture champion forums, functional department meetings, team meetings, individual meetings, social functions and more. We conduct feedback surveys that are designed to help us measure overall staff engagement. These surveys help us assess our culture and obtain feedback on a range of topics that help us build a stronger workplace environment by providing important insight into the areas we may need to focus on. The feedback we obtain through these channels is used to support and measure our progress on key corporate initiatives and adherence to our TRAIT values.
Total Rewards Program
We offer a comprehensive total rewards package that includes competitive pay, health, retirement, time-off and company-ownership opportunities through equity-based incentive programs. Additionally, all our staff members are offered access to well-being services such as financial, legal and estate planning. This year we enhanced our mental health solutions to provide all staff members and their families access to mental wellness tools for meditation, sleep, coaching and personalized therapy sessions. We also offer all staff members a yearly wellness stipend to support their choice of wellness activities. Finally, we have enhanced our family care solutions to provide options for backup child and elder care resources, pet care, tutoring and college coaching. Together, our rewards make up a comprehensive package that helps our staff members live balanced lives, where they can grow personally and professionally.
Commitment to Diversity and Inclusion
Consistent with our TRAIT values, we believe a diverse and inclusive culture supports our ability to serve patients. We recognize there is always more that can be done to promote diversity and inclusion and we actively seek ways to embrace diversity, inclusion and equitable representation at all levels of our organization – and Atara is a much richer place for it.
Atara Staff Diversity
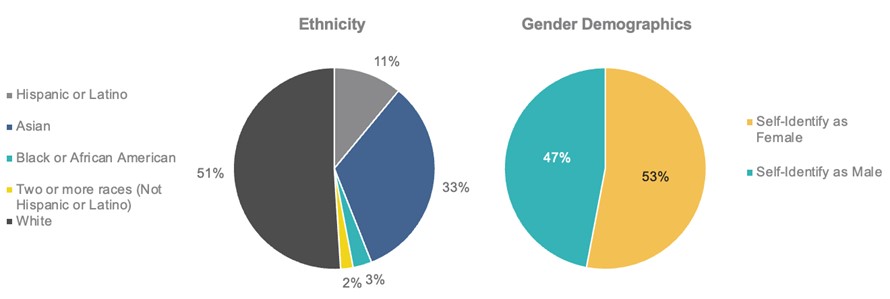
We have established a diversity and inclusion advisory group to promote engagement on a number of topics related to diversity, inclusion & belonging (“DI&B”), including workshops and supporting the emergence of affinity groups as part of our efforts to create a more equitable workplace. We encourage all of our staff to participate in our DI&B initiatives to strengthen and grow our diverse and inclusive culture, which include educational webinars, cultural engagement events, fireside chats and a robust set of DI&B courses available through LinkedIn Learning.
Our first affinity group, which has established the groundwork for future groups, is the Atara Women’s Alliance, the mission of which is to strengthen the leadership, voices and impact of women, and to create an inclusive environment where diverse viewpoints and backgrounds are valued. The Atara Out and Allied affinity group has since been established to help increase
20
visibility, understanding and representation of LGBTQ+ Atara staff and their allies by highlighting & addressing the unique issues facing this community, while providing support and mentoring opportunities to its members.
COVID-19 Considerations
During the continuing COVID-19 pandemic, to support the health, safety and engagement of our staff and business continuity, while also beginning to draw down strict preventive measures and encourage employees to safely return to work, we have enacted numerous safeguards, such as: (i) provided all employees 10 days of COVID-19 supplemental paid sick leave in alignment with Cal-OSHA regulations; (ii) established engineering controls and business processes to reduce risk of on-site transition and preventing “super-spreader” events through physical distancing, and providing our staff with personal protective equipment; (iii) maintained an on-site COVID-19 monitoring program and testing program; and (iv) executed a rapid and transparent communication and education strategy to encourage vaccinations and provide staff with all relevant scientific data at the local, regional and national level.
In 2022, we maintained our Atara COVID-19 Team (“ACT”), sponsored by Company executives, to assess and address business risks associated with the continuing COVID-19 pandemic. The ACT team created clear and regular communications channels, monitored and provided guidance on adapting our approach with local, state and federal laws changes, assessed the impact to our operations, donors, vendors, and clinical trial sites globally, as well as monitored peer companies’ approaches to apply best practices to our processes and decisions that aligned to federal, state and local guidelines.
We continue to work under our “hybrid work” vision adapting several work arrangement models to facilitate, where appropriate, workplace flexibility. In addition to promoting productivity, flexibility and efficiency, we believe these new work arrangement models will reduce the environmental impact of our business and operations and allow us to tap into broader and more diverse talent pools.
We believe our efforts to mitigate the risk of COVID-19 to on-site staff and other corporate stakeholders is a reflection of our commitment to our TRAIT values and to the health and safety of our employees.
Cybersecurity and Data Security
Our enterprise risk management program is periodically reviewed by our leadership team and by our Board. As part of our enterprise risk management program, the Audit Committee regularly considers and discusses our cybersecurity risk exposures and the steps management has taken to monitor and control these exposures, including guidelines and policies designed to mitigate risks identified. The Audit Committee receives periodic reports from our Head of Information Technology regarding our information systems and technology and associated policies, processes and practices for managing and mitigating cybersecurity and technology-related risks.
We maintain policies and procedures that describe our staff’s responsibilities for accessing computerized systems, handling data and information and reporting cybersecurity events in a timely manner. All staff and contractors are required to complete formal training on these policies, which includes ongoing training and learning assessments to inform and train staff and contractors on emerging cybersecurity topics and threats. In addition, Atara has a well-defined incident response plan that provides the framework and guidance to manage through realized risk.
Supplier Code of Conduct
We are committed to maintaining the highest standards of legal and ethical conduct and to reflect our TRAIT values. We expect our suppliers to demonstrate a similar commitment to legal and ethical business practices. Our Supplier Code of Conduct conveys our minimum expectations for our suppliers, and their respective subcontracts and suppliers, to: (i) operate in full compliance with all laws, rules, and regulations; (ii) conduct business ethically and act with integrity; (iii) uphold the human rights of workers and to treat workers with dignity and respect; (iv) provide a safe and healthy working environment; and (v) operate in an environmentally responsible and efficient manner. Our Supplier Code of Conduct can be found on our website at https://www.atarabio.com/suppliers.
Supplier Diversity
We support a diverse and inclusive culture externally through our procurement activities. We utilize small and diverse businesses, including those owned by women, minorities and veterans, to supply goods and services in support of our business and operations.
21
PROPOSAL 2
Advisory Vote on Executive Compensation
Under the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Dodd-Frank Act”), and Section 14A of the Exchange Act, the Company’s stockholders are entitled to vote to approve, on an advisory basis, the compensation of the Company’s named executive officers as disclosed in this Proxy Statement in accordance with SEC rules.
This vote is not intended to address any specific item of compensation, but rather the overall compensation of the Company’s named executive officers and the philosophy, policies and practices described in this Proxy Statement. The compensation of the Company’s named executive officers subject to the vote is disclosed in the Compensation Discussion and Analysis, the compensation tables and the related narrative disclosure contained in this Proxy Statement. As discussed in those disclosures, the Company believes that its compensation policies and decisions are designed to meet two objectives: (i) to attract and retain talented and skilled executives by paying for performance; and (ii) to align compensation of our executives with our stockholders through an appropriate mix of short-term and long-term compensation. Compensation of the Company’s named executive officers is designed to enable the Company to attract and retain talented and experienced executives to lead the Company successfully in a competitive environment.
Accordingly, the Board is asking the stockholders to indicate their support for the compensation of the Company’s named executive officers as described in this Proxy Statement by casting a non-binding advisory vote “FOR” the following resolution:
“RESOLVED, that the compensation paid to the Company’s named executive officers, as disclosed pursuant to Item 402 of Regulation S-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion that accompanies the compensation tables, is hereby APPROVED.”
Because the vote is advisory, it is not binding on the Board, the Compensation Committee or the Company. Nevertheless, the views expressed by the stockholders, whether through this vote or otherwise, are important to the Board and the Compensation Committee, and, accordingly, the Board and the Compensation Committee intend to consider the results of this vote in making determinations in the future regarding executive compensation arrangements.
Advisory approval of this proposal requires the vote of the holders of a majority of the shares present in person, present by remote communication, if applicable, or represented by proxy and entitled to vote on the matter at the annual meeting. Unless the Board decides to modify its policy regarding the frequency of soliciting advisory votes on the compensation of the Company’s named executives, the next scheduled say-on-pay vote will be at the 2024 Annual Meeting of Stockholders.
The Board Of Directors Recommends
A Vote In Favor Of Proposal 2
22
PROPOSAL 3
RATIFICATION OF SELECTION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Audit Committee has selected Deloitte & Touche LLP (“Deloitte & Touche”) as our independent registered public accounting firm for the fiscal year ending December 31, 2023 and has further directed that management submit the selection of its independent registered public accounting firm for ratification by the stockholders at the Annual Meeting. Deloitte & Touche has audited our financial statements since our inception in 2012. Representatives of Deloitte & Touche are expected to be present at the Annual Meeting. They will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Neither our Bylaws nor other governing documents or law require stockholder ratification of the selection of Deloitte & Touche as our independent registered public accounting firm. However, the Audit Committee is submitting the selection of Deloitte & Touche to the stockholders for ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection, the Audit Committee will reconsider whether or not to retain that firm. Even if the selection is ratified, the Audit Committee in its discretion may direct the appointment of different independent auditors at any time during the year if they determine that such a change would be in the best interests of Atara and our stockholders.
The affirmative vote of the holders of a majority of the shares present in person, present by remote communication, if applicable, or represented by proxy and entitled to vote on the matter at the Annual Meeting will be required to ratify the selection of Deloitte & Touche.
Principal Accountant Fees and Services
The following table represents aggregate fees billed to us for each period presented by Deloitte & Touche.
|
|
Fiscal Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
|
|
(in thousands) |
|
|||||
Audit fees(1) |
|
$ |
1,521 |
|
|
$ |
1,272 |
|
Audit-related fees |
|
- |
|
|
- |
|
||
Tax fees |
|
- |
|
|
- |
|
||
All other fees |
|
- |
|
|
|
- |
|
|
Total fees |
|
$ |
1,521 |
|
|
$ |
1,272 |
|
All fees described above were pre-approved by the Audit Committee.
Pre-Approval Policies and Procedures
The Audit Committee has adopted a policy and procedures for the pre-approval of audit and non-audit services rendered by Deloitte & Touche. The policy generally pre-approves specified services in the defined categories of audit services, audit-related services and tax services up to specified amounts. Pre-approval may also be given as part of the Audit Committee’s approval of the scope of the engagement of the independent auditor or on an individual, explicit, case-by-case basis before the independent auditor is engaged to provide each service. The pre-approval of services may be delegated to one or more of the Audit Committee’s members, but the decision must be reported to the full Audit Committee at its next scheduled meeting.
The Audit Committee has determined that the rendering of services other than audit services by Deloitte & Touche is compatible with maintaining the principal accountant’s independence.
THE BOARD OF DIRECTORS AND THE AUDIT COMMITTEE RECOMMEND
A VOTE IN FAVOR OF PROPOSAL 3
23
PROPOSAL 4
AMENDMENT OF THE COMPANY’S CERTIFICATE OF INCORPORATION TO PROVIDE FOR THE EXCULPATION OF OFFICERS AS PERMITTED BY DELAWARE LAW
Background
The Delaware General Corporation Law (the “DGCL”) was amended in 2022 to permit Delaware corporations to exculpate their officers, in addition to their directors, for personal liability in certain actions. After careful consideration, on March [__], 2023, the Board unanimously approved an amendment and restatement of our Amended and Restated Certificate of Incorporation (the “Certificate of Incorporation”) to include the exculpation of officers pursuant to these recent amendments to the DGCL.
As amended, the DGCL only permits, and our proposed amendment to the Certificate of Incorporation would only permit, exculpation of officers for claims that do not involve breaches of the duty of loyalty, acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law, or any transaction in which the officer derived an improper personal benefit. In addition, the exculpation of officers would not apply to claims brought by or in the right of the Company, such as derivative claims.
Rationale for Officer Exculpation Amendment
The rationale for limiting the scope of liability of officers as permitted by Delaware law is to strike a balance between stockholders’ interest in accountability and their interest in the Company’s ability to attract and retain quality officers to work on its behalf. An officer’s role often requires decisions to be made on crucial and time-sensitive matters, opportunities and challenges, which can create a substantial risk of investigations, claims, actions, suits, litigation, costs of defense, and other risks of proceedings seeking to impose liability, regardless of merit, based on hindsight, particularly in light of the current litigious environment in which the Company operates. The Board believes that there is a need for directors and officers to remain free of the risk of financial calamity as a result of an unintentional misstep in connection with their service to the Company. Further, the Board noted that the proposed provision: (i) would not negatively impact stockholder rights, considering the narrow class and type of claims for which officers’ liability would be exculpated, and (ii) conforms to Delaware law.
We expect that many of our peers incorporated in Delaware, with whom we compete for executive talent, will adopt exculpation clauses that limit the personal liability of officers in their certificates of incorporation. Although the proposed amendment to the Certificate of Incorporation is not in response to any specific resignation, threat of resignation or refusal to serve by any officer, we believe a failure to adopt the proposed amendment to the Certificate of Incorporation could impact our recruitment and retention of exceptional officer candidates who may conclude that, without the protection of exculpation, the potential exposure to liabilities, costs of defense and other risks of proceedings exceeds the benefits of serving as an officer of the Company. The proposed provision is not retroactive and is not being proposed in anticipation of any specific litigation, but rather is being proposed on a prospective basis to help mitigate potential future harm to the Company and its stockholders.
Taking into account the limits on the type of claims for which officers’ liability would be exculpated, and the benefits the Board believes would accrue to the Company and its stockholders in the form of an enhanced ability to attract and retain talented officers and the potential to discourage frivolous lawsuits, the Board determined that it is in the best interests of the Company and our stockholders to amend the Certificate of Incorporation as described herein.
Proposed Amendment
The proposed amendments to Article VI of the Certificate of Incorporation are as follows, with added text underlined.
24
The full text of the proposed Second Amended and Restated Certificate of Incorporation is included in Exhibit A to this Proxy Statement.
Required Vote
The affirmative vote of the holders of at least 66 2/3% of the voting power of all of the then-outstanding shares of capital stock of the Company entitled to vote generally in the election of directors, voting together as a single class, is required to authorize the proposed amendment to the Certificate of Incorporation. If this proposal to amend our Certificate of Incorporation is approved by our stockholders, the resulting Amended and Restated Certificate of Incorporation will be filed with the Secretary of State of the State of Delaware shortly after the Annual Meeting. If this proposal to amend our Certificate of Incorporation is not adopted and approved, the current Certificate of Incorporation will remain unchanged.
THE BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT THE STOCKHOLDERS VOTE “FOR” THE AMENDMENT OF THE CERTIFICATE OF INCORPORATION TO PROVIDE FOR THE EXCULPATION OF OFFICERS AS PERMITTED BY DELAWARE LAW.
25
SECURITY OWNERSHIP OF
CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of February 15, 2023, information regarding beneficial ownership of our common stock by:
Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o Atara Biotherapeutics, Inc., 2380 Conejo Spectrum Street, Suite 200, Thousand Oaks, CA 91320.
|
|
Beneficial Ownership(1) |
||
Beneficial Owner |
|
Number of Shares |
|
Percent of Total |
5% Holders: |
|
|
|
|
Redmile Group(2) |
|
9,515,603 |
|
9.9% |
Entities affiliated with BlackRock, Inc.(3) |
|
9,152,149 |
|
9.5% |
Wasatch Advisors(4) |
|
8,391,014 |
|
8.7% |
The Baupost Group(5) |
|
8,123,616 |
|
8.5% |
Entities affiliated with State Street(6) |
|
7,649,740 |
|
8.0% |
Entities affiliated with JPMorgan Chase(7) |
|
7,510,572 |
|
7.8% |
Entities affiliated with The Vanguard Group 1(8) |
|
7,211,048 |
|
7.5% |
Maverick Capital(9) |
|
6,291,013 |
|
6.5% |
Entities affiliated with Goldman(10) |
|
6,108,260 |
|
6.4% |
Directors and Named Executive Officers: |
|
|
|
|
Pascal Touchon, D.V.M.(11) |
|
763,584 |
|
* |
Eric L. Dobmeier(12) |
|
126,000 |
|
* |
Matthew K. Fust(13) |
|
118,500 |
|
* |
Carol Gallagher, Pharm.D.(14) |
|
198,918 |
|
* |
William K. Heiden(15) |
|
118,500 |
|
* |
Ameet Mallik(16) |
|
18,331 |
|
* |
Maria Grazia Roncarolo, M.D.(17) |
|
68,162 |
|
* |
Beth Seidenberg, M.D.(18) |
|
2,002,233 |
|
2.1% |
Jakob Dupont, M.D.(19) |
|
318,926 |
|
* |
Utpal Koppikar(20) |
|
336,290 |
|
* |
Amar Murugan(21) |
|
190,063 |
|
* |
Charlene Banard |
|
— |
|
* |
Eric Hyllengren(22) |
|
127,565 |
|
* |
All Executive Officers and Directors as a Group (13 persons) (23) |
|
4,387,072 |
|
4.5% |
* Represents beneficial ownership of less than 1% of the outstanding common stock.
26
27
28
29
EXECUTIVE OFFICERS
The following table sets forth certain information with respect to our executive officers as of April [__], 2023. Biographical information with regard to Dr. Touchon is presented under “Proposal No. 1—Election of Directors” in this Proxy Statement.
Name |
|
Age |
|
Position(s) |
Pascal Touchon, D.V.M. |
|
60 |
|
President, Chief Executive Officer and Director |
Charlene Banard |
|
59 |
|
Executive Vice President, Chief Technical Officer |
Jakob Dupont, M.D. |
|
58 |
|
Executive Vice President, Global Head of Research and Development |
Eric Hyllengren |
|
48 |
|
Senior Vice President, Chief Financial Officer |
Amar Murugan |
|
48 |
|
Executive Vice President, Chief Legal Officer |
|
|
|
|
|
Charlene Banard, 59, has served as our Executive Vice President, Chief Technical Officer since April 2022. From February 2020 to November 2021, she held the position of Global Head of Technical Operations, Cell & Gene Therapy platform for Novartis Pharmaceuticals Corporation. Prior to Novartis, Ms. Banard was Senior Vice President, Global Quality, Technical Operations at Shire Pharmaceuticals Group PLC from 2013 to 2019, until it was acquired by Takeda Pharmaceutical Company in 2019. She also served in multiple leadership positions at Gilead Sciences, Inc., Cell Genesys, Inc., and Chiron Corporation earlier in her career. Ms. Banard has served as an independent director for Applied Molecular Transport since April 2022. Ms. Banard received her undergraduate degree in biochemistry from the University of California, Davis, and an MBA in Trans-global business from Saint Mary’s College of California.
Jakob Dupont, M.D., 58, has served as our Executive Vice President, Global Head of Research & Development since May 2020. From December 2018 to May 2020, Dr. Dupont served as Chief Medical Officer at Gossamer Bio, Inc. He previously served as the Vice President and Global Head of Breast and Gynecologic Cancer Development for Genentech USA, Inc. (Roche) from January 2017 to December 2018. Before that, Dr. Dupont served as the Senior Vice President and Chief Medical Officer of OncoMed Pharmaceuticals, Inc. from January 2012 to December 2016 and as the Vice President, Clinical Research from October 2011 to January 2012. From September 2006 to October 2011, Dr. Dupont held roles of increasing responsibility in early to late-stage clinical development at Genentech, most recently as its Global Medical Director, Avastin from January 2011, in which capacity he oversaw the global medical strategy and late-stage medical program for Avastin. Dr. Dupont has served as a member of the board of directors of Apexigen, Inc. since August 2020, and as a member of the board of directors of Imugene Ltd., since September 2022. Since February 2009, Dr. Dupont has also served as an adjunct clinical assistant professor at the Stanford University School of Medicine. Prior to joining Genentech in 2006, Dr. Dupont was a faculty member at Memorial Sloan-Kettering Cancer Center from January 2002 to September 2006. Dr. Dupont holds an A.B. in Philosophy from Vassar College, an M.A. in Philosophy from New York University, and an M.D. from the Joan & Sanford I. Weill Medical College of Cornell University.
Eric Hyllengren, 48, has served as our Senior Vice President, Chief Financial Officer since April 2023. Mr. Hyllengren joined the Company in August 2018 as Vice President, Financial Planning and Analysis and added the role of Head of Investor Relations in April 2020. Prior to joining the Company, Mr. Hyllengren spent approximately 15 years at Amgen Inc. in several finance roles with increasing responsibilities where he was most recently Executive Director of Business Development and Head of Alliance Management. Mr. Hyllengren holds a B.B.A. in finance and Russian from the University of Notre Dame and an M.B.A. in finance from the Kellogg School of Management at Northwestern University.
Amar Murugan, 48, has served as our Executive Vice President, Chief Legal Officer since March 2023. Mr. Murugan joined the Company in April 2020 as Senior Vice President, General Counsel. Prior to joining the Company, Mr. Murugan held several senior legal leadership positions at Assertio Therapeutics, Inc. (“Assertio”), a specialty pharmaceutical company, including as Vice President, Legal Affairs from February 2013 to February 2016, as Vice President, Legal and Deputy General Counsel from March 2016 to June 2018, and most recently as Senior Vice President and General Counsel from July 2018 to February 2020. Prior to joining Assertio, he was a partner in the law firms of Baker Botts L.L.P. and McDermott Will & Emery LLP, where he represented technology and life sciences companies in connection with public and private financings, mergers and acquisitions and corporate securities matters. Mr. Murugan holds a B.S. from Georgetown University and a J.D. from the University of California, Los Angeles.
30
EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
This Compensation Discussion and Analysis (“CD&A”) explains the strategy, design, and decision-making related to our compensation programs and practices for our principal executive officer, our principal financial officer and our three other most highly compensated executive officers, referred to collectively as our named executive officers. This CD&A is intended to provide perspective on the information contained in the tables that follow this discussion. For fiscal year 2022, our named executive officers were:
Name |
Position |
Pascal Touchon, D.V.M. |
President and Chief Executive Officer |
Charlene Banard(1) |
Executive Vice President, Chief Technical Officer |
Jakob Dupont, M.D. |
Executive Vice President, Global Head of Research & Development |
Utpal Koppikar(2) |
Executive Vice President, Chief Financial Officer |
Amar Murugan(3) |
Senior Vice President, General Counsel |
While the principal purpose of this CD&A is to discuss the compensation of our named executive officers, many of the programs discussed apply to other members of senior management who, together with the named executive officers, are collectively referred to as our executive officers.
Executive Summary
We are a leading allogeneic T-cell immunotherapy company pioneering the development of transformative therapies for patients with cancer and autoimmune diseases.
In 2022, the Company achieved a significant milestone for our industry, obtaining the first ever regulatory approval in the world for an allogeneic T-cell immunotherapy with the approval of Ebvallo™ by the European Commission (“EC”). As such, we are the most advanced allogeneic T-cell immunotherapy company and intend to rapidly deliver off-the shelf treatments to patients with high unmet medical need. Our platform leverages the unique biology of EBV T-cells and has the capability to treat a wide range of EBV-associated diseases, or other serious diseases through incorporation of engineered chimeric antigen receptors (“CARs”) or T-cell receptors (“TCRs”). We are applying this one platform, which does not require TCR or human leukocyte antigen (“HLA”) gene editing, to create a robust pipeline including: tab-cel for Epstein-Barr virus positive post-transplant lymphoproliferative disease (“EBV+ PTLD”) and other EBV-driven diseases; ATA188, a T-cell immunotherapy targeting EBV antigens as a potential treatment for multiple sclerosis; and multiple next-generation chimeric antigen receptor T-cell (CAR-T) immunotherapies for both solid tumors and hematologic malignancies. Improving patients’ lives is our mission.
31
Our focus on patients and resiliency helped us deliver on key milestones across Atara’s strategic priorities in 2022:
Strategic Priority |
Select Achievements for 2022 |
Tabelecleucel (tab-cel®) |
✓ Announced data from the multi-center Expanded Access Program (“EAP”) in Europe for patients with relapsed or refractory (r/r) EBV+ PTLD following solid organ transplant (“SOT”) or hematopoietic cell transplant (“HCT”) at the 2022 American Society of Clinical Oncology (“ASCO”) Annual Meeting in June 2022; ✓ Executed successful Pre-Approval Inspections (“PAI”) in connection with review of Company’s marketing authorization application (“MAA”) for tab-cel by the European Medicines Agency (“EMA”); ✓ Obtained necessary good manufacturing practices (“GMP”) certificates for facilities in connection with EMA’s review of the Company’s MAA for tab-cel; ✓ Obtained a positive opinion from the Committee for Medicinal Products for Human Use (“CHMP”) of the EMA recommending the EC approve tab-cel as a monotherapy for treatment of adult and pediatric patients two years of age and older with relapsed or refractory EBV+ PTLD who have received at least one prior therapy; for solid organ transplant patients, prior therapy includes chemotherapy unless chemotherapy is inappropriate; ✓ Obtained EC approval of Ebvallo™ (tab-cel) as a monotherapy for treatment of adult and pediatric patients two years of age and older with relapsed or refractory EBV+ PTLD who have received at least one prior therapy; for solid organ transplant patients, prior therapy includes chemotherapy unless chemotherapy is inappropriate; ✓ Amended terms of the commercialization agreement with Pierre Fabre Medicament (“Pierre Fabre”) for tab-cel in Europe to obtain an additional $30M upfront payment upon transfer of the Ebvallo marketing authorization (“MA”) to Pierre Fabre in exchange for reduced royalties and supply price mark-up; ✓ Although the Food and Drug Administration (“FDA”) initially recommended we conduct a clinical study with commercial product as the FDA did not agree that comparability had been demonstrated between product used in the pivotal ALLELE study and the intended commercial product, following further discussions, the FDA recommended a potential path to a biologics license application (“BLA”) submission without the need for a new clinical study; and ✓ Obtained clear guidance and agreement with the FDA on specific chemistry, manufacturing & controls (“CMC”) module 3 requirements for a potential BLA submission. |
ATA 188 |
✓ Completed planned interim analysis (“IA”) of the Phase 2, randomized, placebo-controlled study (“EMBOLD”) in June 2022, including review by the Independent Data and Safety Monitoring Committee (“IDSMC”) of the efficacy, safety and biomarker data available at the data cut off, based on which the IDSMC recommended the study continue without sample size adjustment; ✓ Achieved target enrollment 80 patients for EMBOLD study to enable final read out of the study primary endpoint of confirmed EDSS improvement at 12 months in October 2023; ✓ Presented in March 2022 updated Phase 1 and open-label extension (“OLE”) data that demonstrated 20 out of 24 patients have had either Expanded Disability Status Scale (“EDSS”) improvement or EDSS stability throughout their observation in the study with up to 42 months follow-up; 33% of patients in the high-dose cohorts achieved confirmed EDSS improvement at the 12-month timepoint; ✓ Announced in new magnetic resonance imaging ("MRI") biomarker and OLE clinical data presented as a late-breaking ePoster at the 38th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (“ECTRIMS”) in October 2022; and ✓ Advanced plans for Phase 3 readiness, including interacting with the FDA based on two fast track designations, and further developing our proprietary large-scale bioreactor manufacturing process. |
CAR-T |
✓ Progressed the ATA3219 manufacturing process for scale-up; and ✓ Demonstrated robust activity in ATA3219 preclinical studies. |
32
Compensation Practices and Governance Highlights
The Compensation Committee recognizes that the Company operates in the highly competitive and dynamic biotechnology industry, and in particular, within the cell and gene therapy area. Therefore, in order to provide overall compensation packages to our executive officers that are competitive with the packages offered by companies with which we compete for executive talent, our Compensation Committee reviews market best practices in executive compensation to continually refine our programs. In addition, our Compensation Committee reviews our executive compensation program to evaluate whether our practices align the interests of our directors and executive officers with our stockholders. Below are features of our compensation program which the Compensation Committee believes demonstrate our commitment to link executive compensation to performance and to incentivize the creation of stockholder value.
Pay-for-Performance Philosophy |
❖ We link a significant proportion of the compensation of our named executive officers to the achievement of pre-established corporate goals and stock price appreciation (through use of stock options) |
Stockholder Alignment |
❖ Our emphasis on long-term equity awards aligns the interests of executives and stockholders |
Corporate Strategy Alignment |
❖ Our Compensation Committee establishes incentive compensation programs based on metrics that are designed to be aligned with our corporate strategy and designed to grow stockholder value |
Clawback Policy |
❖ We permit the Company to recoup variable compensation from Section 16 officers, including our named executive officers, in the event of misconduct that causes material accounting restatement |
Change in Control Provisions |
❖ Does not include excessive change in control or severance payments ❖ Provides “double-trigger” change in control benefits ❖ Does not include excise tax gross-ups for severance or change in control benefits |
Stock Ownership Guidelines |
❖ Our stock ownership guidelines for our executive officers and directors further align their interests with those of our stockholders |
Anti-Hedging and Pledging Provisions |
❖ Our Insider Trading Policy strictly prohibits hedging and pledging activities by executive officers and directors |
Repricing Prohibited |
❖ Our equity plans prohibit repricing of underwater stock options without prior stockholder approval |
Stockholder Feedback |
❖ We value the feedback of our stockholders and solicit it regularly throughout the year, including through an annual say-on-pay proposal |
No Guaranteed Annual Bonus or Salary Increase |
❖ We do not guarantee executive officers annual salary increases or bonuses |
Avoid Excessive Perquisites |
❖ Consistent with our pay-for-performance philosophy, we provide very limited perquisites to our executive officers and do not provide personal perquisites such as automobile leases |
Good Standing Requirement |
❖ We require executive officers and all other employees to be in good standing to be eligible for awards under our short-term cash incentive program |
Stockholder Engagement
We believe that strong corporate governance should include year-round, constructive dialogue with stockholders on business strategy, operational execution, corporate governance, our executive compensation program and other critical business matters. Our ongoing engagement with stockholders enables us to better understand stockholders' views and interests, solicit their feedback and share our perspective on these and other important subjects. Our investor relations team and members of our senior management regularly engage and have dialogue with our stockholders throughout the year, including following our quarterly financial releases, through participation at investor conferences and via other channels of communication. These interactions provide an opportunity to discuss and obtain feedback from stockholders regarding their perspectives on the Company’s strategic, operational, governance, executive compensation practices and other topics of interest.
33
Throughout 2022, we engaged with certain stockholders representing approximately 75% of outstanding shares to continue to gain a more thorough understanding of their views and perspectives.
Stockholder Engagement
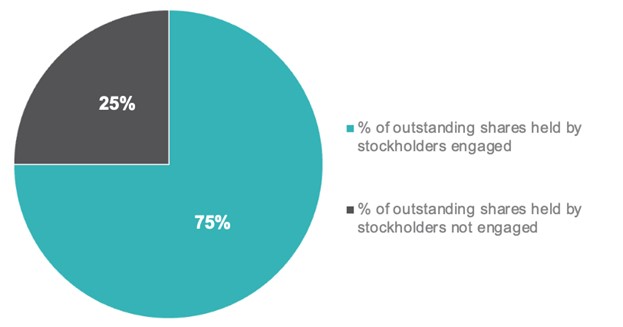
In 2022, key topics discussed with certain stockholders included: (i) the further alignment of executive compensation to performance; (ii) the size of our Board and the number of boards of directors on which certain of our directors serve; (iii) declassification of the Board and supermajority voting requirements for certain changes to Company’s governing documents; (iv) the Company's engagement with employees; and (v) corporate priorities.
The Board, including the Compensation Committee and the Nominating and Corporate Governance Committee, evaluated such stockholder feedback and certain observations made by proxy advisory firms. In response to feedback from stockholders, upon the resignation of two directors in December 2022, the Nominating and Corporate Governance Committee recommended the Board, and the Board approved, a reduction in the size of the Board from ten to eight directors. In addition, based on feedback from stockholders, the Compensation Committee increased the overall proportion of equity awards tied to performance in its assessment of corporate performance against corporate goals further aligning management and stockholder interests.
We also discussed with certain stockholders the Board’s rationale for maintaining the supermajority vote requirement to enact certain changes to the Company’s governing documents and our classified board structure in the context of the biotechnology industry, the charter provisions adopted by similarly positioned companies, the Company’s various initiatives and surveys to engage with its employees and the Board’s objective to create value for stockholders. A significant majority of the stockholders with whom the Company engaged did not object to the Company maintaining such provisions of the Company’s governing documents and our classified board structure. In addition, the Company provided additional disclosure regarding the role of the ad hoc Transactions Committee of the Board in reviewing and advising the Company on potential collaborations, licensing arrangements and other significant corporate transactions.
Consideration of 2022 Say-on-Pay Vote
At our 2022 Annual Meeting of Stockholders, our Say-on-Pay proposal received the support of approximately 97% of the shares voted, which we believe indicates strong support for our compensation program and practices. Our Compensation Committee believes the support for our ongoing efforts to improve and refine our compensation program and further align management and stockholder interests was reflected in this strong support. Based on the level of support for our Say-on-Pay proposal, the Compensation Committee did not make any changes to our executive compensation program in response to the 2022 Say-on-Pay vote.
Compensation Philosophy and Objectives
Our philosophy in setting compensation policies for executive officers has two primary objectives: (i) to attract and retain talented and skilled executives by paying for performance; and (ii) to align compensation of our executives with the interests of our stockholders through an appropriate mix of short-term and long-term compensation. Our Compensation Committee believes that executive compensation should be directly linked to corporate performance and longer-term stockholder value
34
and our executive compensation program is appropriately designed, balanced and appropriately links executive compensation to both continuous and longer-term improvement in corporate performance.
Our compensation program is designed to be consistently and meaningfully focused on pay-for-performance principles, and historically payouts have been both above and below target under our annual incentive plans to reflect Company performance in that year. In determining compensation for executive officers, the Compensation Committee considers multiple factors, including peer group survey data, tenure, role, responsibilities, performance and local competitive market practices and trends. Our Compensation Committee focuses on the following principles to guide decisions regarding executive compensation:
How We Determine Executive Compensation
Role of the Compensation Committee and Executive Officers
The Compensation Committee oversees and approves all compensation arrangements for our executive officers, including our named executive officers. While the Compensation Committee has numerous resources available to it, including input from our Board, Chief Executive Officer, outside legal counsel and independent consultants, ultimate decision-making authority rests with the Compensation Committee. The Compensation Committee retains discretion over base salaries, annual bonuses and equity compensation for executive officers and bases its decision on a careful review of performance as well as the competitive market environment.
The Compensation Committee typically meets at least four times per year, with additional meetings as necessary. The Compensation Committee met six times in 2022. The agenda for each meeting is set by the Chair of the Compensation Committee, with consultation as appropriate with our Chief Executive Officer, Chief People Officer (from 2022 through February 2023), Chief Legal Officer, Chief Financial Officer and Pearl Meyer, the Compensation Committee’s independent compensation consultant. Members of management and Pearl Meyer may be invited to participate in meetings, but the Compensation Committee meets regularly in executive session. Our Chief Executive Officer is often present and participates in discussions regarding compensation of our other executive officers. Executives, including our Chief Executive Officer, are not present during deliberations regarding their own performance or compensation.
35
Role of Compensation Consultants
For the first half of 2022, the Compensation Committee retained the services of Radford as its independent compensation consultant and transitioned to Pearl Meyer effective June 2022. Radford and/or Pearl Meyer assisted the Compensation Committee in its review of executive and director compensation practices, including the market competitiveness of compensation, executive compensation design, benchmarking with industry peers and other technical considerations including those related to tax and accounting.
For 2022 compensation matters, Pearl Meyer advised and assisted with the following:
The Compensation Committee regularly evaluates the services of its consultant and made a determination in June 2022 to transition to Pearl Meyer as its independent compensation consultant. Our Compensation Committee has assessed the independence of Pearl Meyer and Radford, consistent with Nasdaq listing standards and has concluded that the engagement of Pearl Meyer and Radford did not raise any conflict of interest.
Competitive Assessment
A key objective of our executive compensation program is to offer overall compensation packages to our executive officers that are competitive with the packages offered by companies with which we can compete for executive talent in the highly competitive cell therapy talent landscape. The Compensation Committee consults with its independent compensation consultant to develop a peer group of companies to serve as the basis for comparing our executive compensation program to the market.
2022 Peer Group
With the assistance of its independent compensation consultant, the Compensation Committee annually reviews the composition of the peer group to account for changes in both our business and the businesses of the companies in the peer group. While referencing the peer group compensation levels is helpful in determining market-competitive compensation for our named executive officers, the Compensation Committee does not directly tie any pay elements to particular benchmarks within the peer group. Rather, peer data is one consideration, along with employee knowledge, skills and experience, individual performance, and scope of responsibilities, among other factors.
Similar to 2021, the peer group used to determine 2022 compensation consisted of companies with a market capitalization range (at time of determination) of $140 million to $2.5 billion. In developing the peer group, the Compensation Committee considered several key qualitative and quantitative elements as set out below.
36
Following this analysis, the Compensation Committee identified the following 20 publicly-traded, U.S-based biotechnology/pharmaceutical companies as our peer group to be used in reviewing compensation for 2022, which did not change as compared to the 2021 peer group:
Peer Group used to determine 2022 Compensation |
||
Agenus Inc. |
Agios Pharmaceuticals, Inc. |
Allogene Therapeutics Inc. |
Bluebird Biotherapeutics, Inc. |
Clovis Oncology, Inc. |
Editas Medicine, Inc. |
Epizyme, Inc. |
Fate Therapeutics, Inc. |
ImmunoGen, Inc. |
Iovance Biotherapeutics, Inc. |
Karyopharm Therapeutics Inc. |
MacroGenics, Inc. |
Nkarta, Inc. |
Poseida Therapeutics, Inc. |
Precision Biosciences, Inc. |
Rubius Therapeutics, Inc. |
Sangamo Therapeutics Inc. TG |
TCR2 Therapeutics Inc. |
TG Therapeutics, Inc. |
Xencor, Inc. |
|
Following Pearl Meyer’s engagement as the Compensation Committee’s advisor and per the Committee’s normal course agenda, the peer group was reviewed in September 2022. Significant adjustments were made to better align with Atara’s market capitalization at the time. The following revised peer group will be used to evaluate 2023 compensation decisions:
Peer Group used to determine 2023 Compensation |
||
Agenus Inc. |
Alector, Inc.* |
Allogene Therapeutics Inc. |
AlloVir, Inc.* |
Bluebird Biotherapeutics, Inc. |
Clovis Oncology, Inc. |
Fate Therapeutics, Inc. |
ImmunoGen, Inc. |
Instil Bio, Inc.* |
Iovance Biotherapeutics, Inc. |
Karyopharm Therapeutics Inc. |
Kronos Bio, Inc.* |
MacroGenics, Inc. |
Nkarta, Inc. |
Nurix Therapeutics, Inc.* |
Poseida Therapeutics, Inc. |
Precision Biosciences, Inc. |
Rigel Pharmaceuticals, Inc.* |
TCR2 Therapeutics Inc. |
TG Therapeutics, Inc. |
|
* Indicates new peer for 2023 compensation decisions
Elements of Executive Compensation
The Compensation Committee has developed an executive compensation program that consists of the following primary elements:
37
The relative mix of these components is generally weighted towards incentive rather than fixed compensation and towards long-term incentive compared to short-term incentive compensation. We believe this relative weighting towards long-term equity-based compensation further aligns the interests of our executive officers with those of our stockholders.
Base Salary |
Base salaries are set to be competitive within our industry and are important in attracting and retaining talented executives. Base salaries are fixed pay set with consideration for responsibilities, market data, employee knowledge, skills and experience, individual performance and scope of responsibilities, among other factors. |
Annual Cash Bonus |
The annual cash incentive award plan is intended to motivate and reward our executives for the achievement of individual goals as well as the strategic goals of the Company.
In 2022, our annual incentives were based on key strategic, research, development, regulatory, clinical, financial and operational corporate objectives. |
Long-Term Equity Incentives |
Long-term equity awards are designed to incentivize executives to deliver long-term stockholder value, while also providing a retention vehicle for our top executive talent.
Equity awards in 2022 were delivered as a combination of the following: • Service-based stock options (50%) • Performance-based stock options (10%) • RSUs (40%)
The value of these awards depends on the performance of our common stock price, in order to align employee and executive interests with those of our stockholders over the longer term. |
Target Compensation Mix
The Compensation Committee conducts an annual performance and compensation review for our executive officers, including our Chief Executive Officer. The Compensation Committee reviews base salary, annual bonus and equity-based compensation annually as part of this review. The Compensation Committee has not established formal policies or guidelines for allocating compensation between annual and long-term incentive compensation, or between cash and non-cash compensation. Instead, through our compensation program, the Compensation Committee seeks to align pay and performance. As can be seen in the graphs below, a large percentage of executive pay in 2022 was variable and “at-risk” (90% for the Chief Executive Officer and 85% on average for our other named executive officers), meaning that value to the executive is tied directly to corporate goals, individual goals (except for the Chief Executive Officer) and stock price performance. In this sense, we believe we have established a pay-for-performance culture and pay program. The balance between these components may change from year to year based on corporate strategy and objectives, among other considerations.
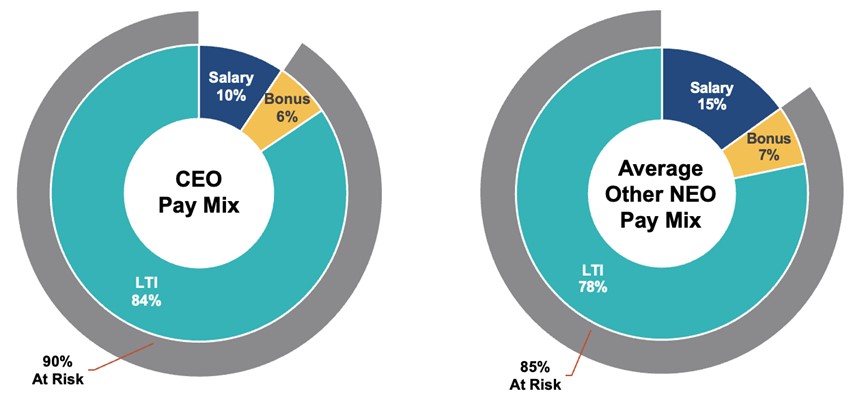
38
Base Salary
In considering the appropriate level of base salaries for our named executive officers for 2022, the Compensation Committee employed a holistic analysis of multiple relevant factors using its professional judgment and experience, emphasizing the following:
The Compensation Committee considered the base salaries of similarly situated executives at our peer group companies for an understanding of whether our compensation program is competitively positioned to retain our highly qualified named executive officers. The Compensation Committee determined that each then-serving named executive officer should receive an increase in his or her base salary for 2022 as set forth below. For Ms. Banard, the Compensation Committee set her base salary at the time of her hire based on its review of competitive market data, including that of our peer group companies.
Named Executive Officer |
2021 Base Salary |
2022 Base Salary |
Year-over-Year |
Pascal Touchon, D.V.M. |
$682,500 |
$723,450 |
6.0% |
Charlene Banard(1) |
N/A |
$430,000 |
N/A |
Jakob Dupont, M.D. |
$520,000 |
$546,000 |
5.0% |
Utpal Koppikar |
$475,800 |
$504,348 |
6.0% |
Amar Murugan(2) |
$431,600 |
$515,000 |
19.3% |
Annual Cash Bonuses
Annual Performance Goals
The Compensation Committee works with the executive team to develop goals with respect to the Company’s annual incentive compensation program and ultimately recommends a list of goals to the Board for approval. The Board reviews the strategic, operational and financial components of the goals and approves the goals as well as a weighting for each goal based on its relative importance. Our Chief Executive Officer and executive team provide updates to the Board through the course of the year on performance towards these goals. At the end of the year, our Chief Executive Officer presents the Compensation Committee with a proposed score based on the Company’s performance against the goals. After discussion and review, the Compensation Committee makes a recommendation on the overall corporate achievement score compared to the annual performance goals to the Board for approval. This score is then used to establish the corporate portion of annual bonus payments.
In addition, our Chief Executive Officer works with each executive officer to establish individual performance goals and objectives. Individual goals are evaluated in a more qualitative and subjective manner than the corporate goals, and executive officers are evaluated on overall achievement of individual goals as well as overall contribution to the Company’s corporate goals. This evaluation is performed by our Chief Executive Officer who then recommends an individual bonus amount to the Compensation Committee for consideration and approval.
39
Annual Bonus Process
As noted above, the Compensation Committee conducts an annual performance and compensation review for our executive officers, including our Chief Executive Officer. The review is typically conducted over a series of meetings beginning at the end of the year as part of the Company’s broader annual performance review process. The annual corporate score which is determined as described above in “Annual Performance Goals” is used to determine the size of the Company-wide bonus pool. Our Chief Executive Officer receives a bonus based entirely on corporate performance since our Chief Executive Officer has ultimate operational responsibility for corporate performance. Our other executives receive a bonus which is primarily linked to corporate performance (85%), with a smaller individual performance component (15%). The individual performance component of the bonus for these executive officers can be modified up to 125% or down to 0% dependent on performance in the prior year. In addition, the Compensation Committee retains flexibility to increase or decrease any and all compensation components to reflect performance.
2022 Bonus Targets
Bonus targets (expressed as a percentage of base salary) are reviewed annually by the Compensation Committee, taking into consideration competitive market data, and adjusted if deemed appropriate by the Compensation Committee. The 2022 target bonuses, as a percentage of base salary determined by level, have not changed since 2020. Ms. Banard’s target bonus was established at the time she joined the Company based on its review of competitive market data, including our peer group companies, as well as the Company’s internal pay practices.
Named Executive Officer |
Target Bonus |
Target Bonus ($ amount) |
Pascal Touchon, D.V.M. |
65% |
$470,243 |
Charlene Banard |
45% |
$132,534(1) |
Jakob Dupont, M.D. |
45% |
$245,700 |
Utpal Koppikar |
45% |
$226,957 |
Amar Murugan |
40% |
$206,000 |
2022 Corporate Goal Development, Weighting and Performance Evaluation
In February 2022, the Board reviewed and approved the corporate goals and objectives for 2022, as summarized in the table below. In selecting these goals, the Compensation Committee wanted to strike the appropriate balance between continuing to build out our enterprise capabilities and our development programs: ATA188, tab-cel and CAR-T. At the time the 2022 corporate goals were set, the Board and management believed that such goals were challenging, and that achieving them would require not only continued strong progress on research and clinical development, manufacturing and operational capabilities, and prudent fiscal and legal management, but also a high level of effort and execution on the part of our named executive officers.
40
The Board also applied a performance weighting to such goals relative to our overall performance to reflect the prioritization of key business objectives. Additionally, a weighting between corporate performance and individual performance was applied for each named executive officer (other than the Chief Executive Officer) to reflect the level of impact such individual would be able to make on the overall corporate performance. As noted above, the Chief Executive Officer’s annual bonus was entirely based on corporate performance. The relative weighting between our 2022 corporate objectives relating to advancing key assets in our pipeline and enhancing our enterprise capabilities is summarized below.
2022 Objectives |
2022 Goals Summary |
Results |
Weight |
ATA188 |
• Complete planned IA in Q2 • Enroll 80 patients in the EMBOLD study by end of July 2022 • Reach certain manufacturing milestones and complete all GMP readiness activities by end of 2022 |
• Completed planned IA in June 2022 • Achieved target enrollment 80 patients for the EMBOLD study to enable final read out of the study primary endpoint of confirmed EDSS improvement at 12 months in October 2023 • Further developed our proprietary large-scale bioreactor manufacturing process |
40% |
Tab-cel |
• Complete Submission of BLA for EBV+ PTLD in Q2 2023 • Complete EMA PAI in first half of 2022 • Achieve CHMP recommendation for approval in second half of 2022 • Progress critical and cross-functional US and EU launch readiness activities by end of 2022 |
• Executed successful PAIs and obtained necessary GMP certificates in connection with review of Company’s MAA by the EMA for tab-cel • Obtained a positive CHMP opinion of the EMA recommending the EC approve tab-cel • Obtained EC approval of Ebvallo™ (tab-cel) • Amended terms of the commercialization agreement with Pierre Fabre for Ebvallo in Europe to obtain an additional $30M upfront payment upon transfer of the Ebvallo MA to Pierre Fabre in exchange for reduced royalties and supply price mark-up • Although the FDA initially recommended we conduct a clinical study with commercial product as the FDA did not agree that comparability had been demonstrated between product used in the pivotal ALLELE study and the intended commercial product, following further discussions, the FDA recommended a potential path to a BLA submission |
25% |
41
|
|
without the need for a new clinical study • Obtained clear guidance and agreement on specific CMC module 3 requirements for a potential BLA submission • Supported EU readiness activities to enable commercialization of Ebvallo™ by Pierre Fabre following approval of the MAA for tab-cel by the EC |
|
CAR-T |
• Submit ATA3219 Investigational New Drug Application (“IND”) in Q4 • Deliver ATA3271 IND package to partner in Q4 |
• Although an IND for ATA3219 was not filed in 2022, we progressed the ATA3219 manufacturing process for scale-up • Demonstrated robust activity in ATA3219 preclinical studies • Bayer AG (“Bayer”), our collaboration partner for ATA2271 and ATA3271, terminated our collaboration for these programs in July 2022, in connection with which the Company received $4.2M from Bayer |
20% |
Enterprise Capabilities |
• Enable successful organizational and cultural transition to improve prioritization, resource allocation, and business continuity • Develop and implement processes and procedures designed to encourage ethical and compliant commercial practices • Meet Company’s cash runway target |
• Executed and consummated long-term strategic agreement with FUJIFILM Diosynth Biotechnologies, California, Inc. (“FDB”), a subsidiary of FUJIFIM Corporation under which FDB acquired the Company’s T-Cell Operations and Manufacturing (“ATOM”) facility in Thousand Oaks, California for $100M upfront, retaining manufacturing and quality staff at the facility • Entered into a royalty interest financing agreement totaling $31 million with HealthCare Royalty (“HCRx”) for Ebvallo™ in Europe and other territories covered by the Company’s commercialization agreement with Pierre Fabre • Amended terms of the commercialization agreement with Pierre Fabre for Ebvallo in Europe with Pierre Fabre to obtain an |
15% |
42
|
|
additional $30M upfront payment upon transfer of the Ebvallo MA to Pierre Fabre in exchange for reduced royalties and supply price mark-up • Developed and implemented processes and procedures adapted to Company’s evolving business strategy and with regard to prioritization, resource allocation, business continuity |
|
We achieved several key corporate goals and objectives set for 2022, including, but not limited to: (i) obtaining a first of its kind approval by the EC of Ebvallo™ as a monotherapy for treatment of adult and pediatric patients two years of age and older with relapsed or refractory EBV+ PTLD who have received at least one prior therapy (for solid organ transplant patients, prior therapy includes chemotherapy unless chemotherapy is inappropriate); (ii) completing the IA of the EMBOLD study in June 2022, including review by IDSMC of the efficacy, safety and biomarker data available at the data cut off, based on which the IDSMC recommended the EMBOLD study continue without sample size adjustment; and (iii) achieving target enrollment of 80 patients for EMBOLD study to enable final read out of the EMBOLD study primary endpoint (confirmed EDSS improvement at 12 months) in October 2023.
We also made significant progress on our key strategic priorities, including with respect to corporate goals that were not met. For example, with regard to tab-cel in the U.S., despite the challenges encountered in our discussions with the FDA for a potential BLA submission for tab-cel and the FDA’s initial recommendation that we conduct a clinical study with commercial product as the FDA did not agree that comparability was demonstrated between product used in the pivotal ALLELE study and the intended commercial product, following further discussions, the FDA recommended a potential path to a BLA submission without the need for a new clinical study. Through our continued engagement with the FDA, we also obtained clear guidance and agreement with the FDA on specific CMC module 3 requirements for a potential BLA submission. Similarly, although we were not able to file an IND for ATA3219 in 2022, we progressed the ATA3219 manufacturing process for scale-up and demonstrated robust activity in ATA3219 preclinical studies.
During the course of 2022, we announced important data that supports the potential value of our key programs to patients and the Company. In March 2022, we presented updated ATA188 Phase 1 and OLE data that demonstrated 20 out of 24 patients have had either EDSS improvement or EDSS stability throughout their observation in the study with up to 42 months follow-up and that 33% of patients in the high-dose cohorts achieved confirmed EDSS improvement at the 12-month timepoint. In June 2022, we presented data from the multi-center EAP in Europe for patients with relapsed or refractory (r/r) EBV+ PTLD following SOT or HCT at the 2022 ASCO meeting. In October 2022, we announced new MRI biomarker and OLE clinical data presented as a late-breaking ePoster at the ECTRIMS Congress.
We took significant steps and executed several complex transactions in 2022 to extend our cash runway through non-dilutive means. We executed and consummated a long-term strategic agreement with FDB under which FDB acquired the Company’s ATOM facility for an upfront payment of $100 million while securing a long-term manufacturing agreement with FDB. We also entered into a royalty interest financing agreement totaling $31 million with HCRx for Ebvallo™ in Europe and other territories covered by the Company’s commercialization agreement with Pierre Fabre. Finally, we amended the terms of the commercialization agreement with Pierre Fabre for Ebvallo™ in Europe to obtain an additional $30 million upfront payment upon transfer of the Ebvallo™ MA to Pierre Fabre in exchange for reduced royalties and supply price mark-up. Altogether, these transactions, coupled with the Company’s actions in 2022 directed towards improving its operating efficiency, resulted in an extension of its cash runway into Q2 2024.
Based on the Board’s evaluation of the Company’s foregoing results and achievements in 2022 and considering the continuing macro challenges and uncertainty, and as recommended by the Compensation Committee, the Board approved a 75% achievement of the 2022 corporate goals.
43
In consideration of the foregoing, in February 2023, pursuant to the terms and conditions of the applicable annual bonus guidelines, the Compensation Committee approved annual cash bonus payments for each named executive officer as reflected in the table below.
Name |
2022 |
Corporate Score Modifier |
2022 Individual Modifier |
2022 Actual Bonus Paid as a % of Salary |
2022 Actual |
Pascal Touchon, D.V.M. |
$470,243 |
75% |
-- |
48.7% |
$349,611 |
Jakob Dupont, M.D. |
$245,700 |
75% |
115% |
36.4% |
$197,559 |
Utpal Koppikar |
$226,957 |
75% |
125% |
37.1% |
$185,609 |
Amar Murugan |
$206,000 |
75% |
125% |
32.9% |
$150,389 |
Charlene Banard(2) |
$132,534 |
75% |
100% |
34.5% |
$102,564 |
Long-Term Incentives
Our goal is to align executive compensation and performance that advances our critical business objectives. Therefore, a significant portion of the named executive officers’ total compensation typically has consisted of, and is expected to continue to consist of, equity-based awards. In evaluating the mix of equity awards for 2022, the Compensation Committee considered market trends, as well as applicable feedback from stockholders and proxy advisory firms, and determined that a mix of service-based stock options, RSUs and performance-based stock options would be the most appropriate incentive structure for our named executive officers to reward performance over time and achieve our retention objectives. Ms. Banard’s new hire equity grant was established at the time she joined the Company based on the Compensation Committee’s review of competitive market data, including our peer group companies, as well as the Company’s internal pay practices. The following table outlines the equity awards granted to named executive officers in 2022.
Equity Grants
Named Executive Officer |
Grant Date |
Type of Grant |
Stock Options (#) |
Performance Options (#) |
RSUs |
Pascal Touchon, D.V.M. |
March 1, 2022 |
Annual |
339,419 |
77,140 |
180,565 |
|
|
|
|
|
|
Jakob Dupont, M.D. |
March 1, 2022 |
Annual |
147,573 |
33,539 |
78,506 |
|
|
|
|
|
|
Utpal Koppikar |
March 1, 2022 |
Annual |
115,107 |
26,160 |
61,235 |
|
|
|
|
|
|
Amar Murugan |
March 1, 2022 |
Annual |
97,398 |
22,136 |
51,814 |
|
|
|
|
|
|
Charlene Banard |
April 25, 2022 |
New Hire |
292,023 |
33,184 |
155,350 |
44
Stock Options (50% of total grant value)
The Compensation Committee grants stock options to emphasize retention and align the named executive officers’ interests with those of stockholders. We believe that stock options provide a necessary balance between performance-based and time-vesting awards as the stock options are considered by us to be performance-based because the executive officers will realize no value unless the stock price appreciates from the date of grant. Stock option awards vest monthly and will generally be fully vested four years after the option grant date, subject to continuous service.
Performance-Vested Stock Options (10% of total grant value)
Following feedback from our stockholders and the Compensation Committee’s desire to drive the senior executive team to execute on a specific goal related to ATA188, a performance-vested component was added to the Company’s equity program for 2022. Performance-vested options vest as follows:
Although a minority practice amongst our peers, the Compensation Committee felt it important to respond to stockholder feedback and establish a focus on objectives related to ATA188. The Committee will continue to consider the use of performance-vested equity in future compensation cycles given their ability to align executive and stockholder interests, as is the case with time-vested stock options and RSUs.
RSUs (40% of total grant value)
RSUs are viewed as an important retention vehicle for named executive officers, as well as a variable and at-risk component of executive compensation. Market trends reflect their favored use among our peer group and other companies in the biotechnology sector. RSU awards vest quarterly and will generally be fully vested four years after the RSU grant date, subject to continuous service.
Other Compensation Policies and Practices
Insider Trading Policy (including Anti-Hedging and Anti-Pledging)
Our Insider Trading Policy prohibits all employees (including executive officers) and directors from: (i) buying or selling our stock while aware of any material nonpublic information; (ii) buying or selling our stock while our trading window is closed; and (iii) engaging in short sales, transactions in put or call options, hedging transactions, margin accounts, pledges or other inherently speculative transactions with respect to our stock at any time.
Stock Ownership Guidelines
Effective as of January 1, 2021, in order to further align their financial interests with those of our stockholders as well as to promote sound corporate governance, we adopted stock ownership guidelines for our named executive officers. These guidelines establish the following ownership targets for the value of stock held by each individual:
Role |
Ownership Guideline |
Chief Executive Officer |
3x |
All other Officers |
1x |
Non-employee Directors |
3x |
45
The guidelines must be achieved (i) by each individual covered under the guidelines (a “Covered Individual”) by December 31, 2025 and (ii) for any individual who becomes a Covered Individual after January 1, 2021, by December 31 of the calendar year in which the fifth anniversary of the date the individual became a Covered Individual occurs. The shares counted towards the ownership requirements specified in the guidelines include shares owned outright (valued at fair market value), unvested RSUs (valued at 70% of fair market value) and vested stock options (valued at 70% of intrinsic value, the number of shares vested multiplied by the difference between the exercise price and the Nasdaq closing price of our common stock on the measurement date), as applicable, to account for any sales of such equity required to be sold or withheld to cover taxes.
Once a Covered Individual has complied with these Guidelines, such Covered Individual shall be deemed to remain compliant under the guidelines notwithstanding any change in his or her base pay or change in the value of his or her equity which would otherwise make such Covered Individual non-compliant; provided that a sale or other disposition of shares of stock (other than the sale, or surrender to the Company, of shares of stock in payment of the exercise price of any equity award or in satisfaction of taxes in connection with vesting or exercise of any equity award) may, as applicable, cause the Covered Individual to no longer be deemed in compliance with the guidelines. A Covered Individual’s ownership is reviewed annually as of December 31 of a calendar year to determine if the Covered Individual has met, or is on track to meet, the applicable guideline.
As of December 31, 2022, each Covered Individual is either in compliance, or on track to be in compliance, with the ownership targets set forth in the guidelines within the timeframe specified in the guidelines.
Clawback Policy
Our Clawback Policy provides for the Company’s recoupment of certain incentive compensation paid to covered officers of the Company under certain circumstances of misconduct. The Board may delegate determinations to be made under the Clawback Policy to a committee of the Board.
Pursuant to the Clawback Policy: (i) “misconduct” refers to a knowing violation of SEC rules or regulations or Company policy or the willful commission of an act of fraud, dishonesty or gross recklessness in the performance or disregard of a person’s duties; and (ii) “covered officer” refers to a current or former officer of the Company who is or was designated as an “officer” for purposes of Section 16 of the Securities Exchange Act of 1934, as amended, by the Board or an appropriate committee of the Board.
There was no recoupment under the Clawback Policy in 2022.
Accounting and Tax Considerations
Under the Financial Accounting Standard Board (“FASB”) Accounting Standards Codification (Topic 718) (“ASC 718”), we are required to estimate and record an expense for each award of equity compensation (including stock options, RSUs and performance stock options) over the vesting period of the award. For such period as stock options, RSUs and performance stock options remain the sole components of our long-term compensation program, we expect to record stock-based compensation expense on an ongoing basis according to ASC 718. Compensation expense relating to awards subject to performance conditions is recognized if it is probable that the performance goals will be achieved. The probability of achievement of such goals is assessed on a quarterly basis.
Compensation Risk Assessment
The Compensation Committee has reviewed the Company’s compensation policies and practices, in consultation with Pearl Meyer, to assess whether they encourage employees to take inappropriate risks. After reviewing and assessing the Company’s compensation philosophy, terms and practices, including the mix of fixed and variable, short and long-term incentives and overall pay, incentive plan structures and the checks and balances built into, and oversight of, each plan and practice, the Compensation Committee determined that any risks arising from our compensation policies and practices for our employees are not reasonably likely to have a material adverse effect on our Company as a whole and strike an appropriate balance between short-term and long-term compensation and incentivizes our executives to act in a manner that prudently manages enterprise risk.
46
Compensation Committee Report2
The Compensation Committee, consisting solely of independent directors, has reviewed and discussed with management the Compensation Discussion and Analysis (“CD&A”) contained in this Proxy Statement. Based on this review and discussion, the Compensation Committee has recommended to the Board that the CD&A be included in this Proxy Statement and incorporated into the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022.
Eric L. Dobmeier
William K. Heiden
Ameet Mallik
2 The material in this report is not “soliciting material,” is being furnished and shall not be deemed “filed” with the Commission and is not to be incorporated by reference in any filing of Atara under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
47
CEO Pay Ratio Disclosure
Under the Dodd-Frank Act and the related SEC rule (the “Rule”), we are required to provide our stockholders with specified disclosure regarding the relationship of our Chief Executive Officer’s total compensation to the total compensation of our median employee, referred to as “pay-ratio” disclosure. For 2022, the annual total compensation of our Chief Executive Officer, for purposes of this disclosure, was $5,158,127, and the compensation of our median employee was $254,363, resulting in a pay ratio of approximately 20:1.
In accordance with SEC rules, we have identified the median employee as of December 31, 2022 by: (i) aggregating for each applicable employee: (a) annual base salary for salaried employees (or annual scheduled wages plus overtime for hourly employees), (b) the target incentive pay paid for fiscal year 2022, and (c) the estimated grant date fair value of any equity awards granted during fiscal year 2022, and (ii) ranking this compensation measure for our employees from lowest to highest. This calculation was performed for all employees employed by us as of December 31, 2022, excluding the Chief Executive Officer, whether employed on a full-time, part-time or seasonal basis. In making this determination, we annualized the compensation of employees who were employed by the Company for less than the entire fiscal year. This compensation measure was consistently applied to all employees included in the calculation and reasonably reflects the annual compensation of employees. All amounts paid in currencies other than US Dollars were converted to US Dollars based on the applicable average annual exchange rates.
We believe that the pay ratio reported above is a reasonable estimate calculated in a manner consistent with SEC rules based on our internal records and the methodology described above. The SEC rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices. Therefore, the pay ratio reported by other companies may not be comparable to the pay ratio reported above, as other companies have different employee populations and compensation practices and may utilize different methodologies, exclusions, estimates and assumptions in calculating their own pay ratios.
Name |
Year |
Salary |
Bonus |
Stock |
Option |
All Other |
Total |
Pascal Touchon, D.V.M. |
2022 |
717,938 |
349,611 |
1,843,569 |
2,236,703 |
10,306 |
5,158,127 |
48
2022 Summary Compensation Table
The following table sets forth information regarding compensation awarded to or earned by our named executive officers listed below during the year ended December 31, 2022 and, to the extent required by SEC disclosure rules, 2021 and 2020.
Name and Principal Position |
Year |
Salary ($) |
Bonus ($)(1) |
Stock Awards ($)(2) |
Option Awards ($)(3) |
Non-Equity Incentive Plan Compensation |
All Other Compensation ($)(5) |
Total ($) |
Pascal Touchon, D.V.M. President & Chief Executive Officer |
2022 2021 2020 |
717,938 680,625 654,857 |
--- --- --- |
1,843,569 2,004,484 2,235,600 |
2,236,703 2,004,525 1,959,208 |
349,611 418,356 422,500 |
10,307 9,345 13,662 |
5,158,127 5,117,335 5,285,827 |
|
|
|
|
|
|
|
|
|
Charlene Banard(6) EVP, Chief Technical Officer |
2022 |
297,693 |
50,000 |
1,132,502 |
1,415,289 |
102,564 |
203,700 |
3,201,748 |
|
|
|
|
|
|
|
|
|
Jakob Dupont, M.D. EVP, Global Head of Research and Development |
2022 2021 2020 |
542,500 519,231 319,231 |
--- 130,000 130,000 |
801,546 921,600 443,075 |
972,477 921,622 1,757,296 |
197,559 227,963 143,346 |
10,471 7,853 3,297 |
2,524,553 2,728,269 2,796,245 |
|
|
|
|
|
|
|
|
|
Utpal Koppikar EVP, Chief Financial Officer |
2022 2021 2020 |
500,505 475,096 460,947 |
--- |
625,209 774,138 885,431 |
758,532 774,155 597,073 |
185,609 188,248 187,032 |
13,807 13,045 7,470 |
2,083,662 2,224,682 2,137,953 |
|
|
|
|
|
|
|
|
|
Amar Murugan(7) EVP, Chief Legal Officer |
2022 2021 |
457,328 430,962 |
--- |
529,021 714,236 |
641,833 714,252 |
150,389 170,760 |
10,807 10,042 |
1,789,378 2,040,252 |
49
2022 Grants of Plan-Based Awards
The following table provides information regarding grants of plan-based awards to our named executive officers for the fiscal year ended December 31, 2022:
Name |
Award Type |
Grant Date |
Estimated Future Payouts Under Non-Equity Incentive Plan Awards (1) |
Estimated Future Payouts under Equity Incentive Plan Awards |
All Other |
All Other |
Exercise |
Grant |
||||
Threshold ($) |
Target ($) |
Maximum ($) |
Threshold ($) |
Target (#)(4) |
Maximum (#)(4) |
|||||||
(a) |
|
(b) |
(c) |
(d) |
(e) |
(f) |
(g) |
(h) |
(i) |
(j) |
(k) |
(l) |
Pascal |
Annual RSU Grant |
3/1/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
180,565 |
--- |
--- |
1,843,569 |
Touchon, |
Annual Option Grant |
3/1/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
339,419 |
10.21 |
2,236,703 |
D.V.M. |
Annual Cash |
--- |
--- |
470,243 |
(2) |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
|
PSO (4) |
--- |
--- |
--- |
--- |
--- |
77,140 |
77,140 |
--- |
--- |
--- |
--- |
Charlene |
New Hire RSU Grant |
4/25/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
155,350(5a) |
--- |
--- |
1,132,502 |
Banard |
New Hire Option Grant |
4/25/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
292,023(6a) |
7.29 |
1,415,289 |
|
Annual Cash |
--- |
--- |
132,534 |
(3) |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
|
PSO (4) |
--- |
--- |
--- |
--- |
--- |
33,184 |
33,184 |
--- |
--- |
--- |
--- |
Jakob Dupont, |
Annual RSU Grant |
3/1/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
78,506 |
--- |
--- |
801,546 |
M.D. |
Annual Option Grant |
3/1/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
147,573 |
10.21 |
972,477 |
|
Annual Cash |
--- |
--- |
245,700 |
(3) |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
|
PSO (4) |
--- |
--- |
--- |
--- |
--- |
33,539 |
33,539 |
--- |
--- |
--- |
--- |
Utpal Koppikar |
Annual RSU Grant |
3/1/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
61,235 |
--- |
--- |
625,209 |
|
Annual Option Grant |
3/1/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
115,107 |
10.21 |
758,532 |
|
Annual Cash |
--- |
--- |
226,957 |
(3) |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
|
PSO (4) |
--- |
--- |
--- |
--- |
--- |
26,160 |
26,160 |
--- |
--- |
--- |
--- |
Amar Murugan |
Annual RSU Grant |
3/1/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
51,814 |
--- |
|
529,021 |
|
Annual Option Grant |
3/1/2022 |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
97,398 |
10.21 |
641,833 |
|
Annual Cash |
--- |
--- |
206,000 |
(3) |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
|
PSO (4) |
--- |
--- |
--- |
--- |
--- |
22,136 |
22,136 |
--- |
--- |
--- |
--- |
50
Outstanding Equity Awards as of December 31, 2022
The following table provides information regarding outstanding equity awards held by our named executive officers as of December 31, 2022.
|
|
|
Option Awards |
Stock Awards |
|
|
|
||||
Name |
Grant Date |
Notes |
Number of Securities Underlying Unexercised Options Exercisable (#) |
Number of Securities Underlying Unexercised Options Unexercisable (#) |
Option Exercise Price ($/sh) |
Option Expiration Date |
Number of Shares or Units That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($)(1) |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise |
Option Expiration Date |
Pascal |
06/24/2019 |
(2) |
--- |
--- |
--- |
--- |
28,125 |
92,250 |
--- |
--- |
--- |
Touchon, |
06/24/2019 |
(3) |
196,875 |
28,125 |
20.43 |
06/23/2029 |
--- |
--- |
--- |
--- |
--- |
D.V.M. |
03/01/2020 |
(4) |
--- |
--- |
--- |
--- |
46,875 |
153,750 |
--- |
--- |
--- |
|
03/01/2020 |
(5) |
166,375 |
75,625 |
12.15 |
02/28/2030 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2021 |
(4) |
--- |
--- |
--- |
--- |
65,919 |
216,214 |
--- |
--- |
--- |
|
03/01/2021 |
(5) |
77,388 |
99,498 |
17.11 |
02/28/2031 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2022 |
(4) |
--- |
--- |
--- |
--- |
146,710 |
481,209 |
--- |
--- |
--- |
|
03/01/2022 |
(5) |
63,641 |
275,778 |
10.21 |
02/28/2032 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2022 |
(6) |
--- |
--- |
--- |
--- |
--- |
--- |
77,140 |
10.21 |
02/28/2032 |
Charlene |
04/25/2022 |
(7) |
--- |
--- |
--- |
--- |
155,350 |
509,548 |
--- |
--- |
--- |
Banard |
04/25/2022 |
(3) |
--- |
292,023 |
7.29 |
04/24/2032 |
--- |
--- |
--- |
--- |
--- |
|
04/25/2022 |
(6) |
--- |
--- |
--- |
--- |
--- |
--- |
33,184 |
7.29 |
04/24/2032 |
Jakob |
05/14/2020 |
(7) |
--- |
--- |
--- |
--- |
17,348 |
56,901 |
--- |
--- |
--- |
Dupont, |
05/14/2020 |
(8) |
159,219 |
98,281 |
9.58 |
05/13/2030 |
--- |
--- |
--- |
--- |
--- |
M.D. |
03/01/2021 |
(4) |
--- |
--- |
--- |
--- |
30,310 |
99,417 |
--- |
--- |
--- |
|
03/01/2021 |
(5) |
35,581 |
45,746 |
17.11 |
02/28/2031 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2022 |
(4) |
--- |
--- |
--- |
--- |
63,788 |
209,225 |
--- |
--- |
--- |
|
03/01/2022 |
(5) |
27,670 |
119,903 |
10.21 |
02/28/2032 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2022 |
(6) |
--- |
--- |
--- |
--- |
--- |
--- |
33,539 |
10.21 |
02/28/2032 |
Utpal |
06/07/2018 |
(3) |
75,000 |
--- |
42.30 |
06/06/2025 |
--- |
--- |
--- |
--- |
--- |
Koppikar |
02/06/2019 |
(9) |
--- |
--- |
--- |
--- |
4,265 |
13,989 |
--- |
--- |
--- |
|
02/06/2019 |
(5) |
32,703 |
1,422 |
38.47 |
02/05/2029 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2020 |
(4) |
--- |
--- |
--- |
--- |
11,531 |
37,822 |
--- |
--- |
--- |
|
03/01/2020 |
(4) |
--- |
--- |
--- |
--- |
9,375 |
30,750 |
--- |
--- |
--- |
|
03/01/2020 |
(5) |
50,703 |
23,047 |
12.15 |
02/28/2030 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2021 |
(4) |
--- |
--- |
--- |
--- |
25,462 |
83,515 |
--- |
--- |
--- |
|
03/01/2021 |
(5) |
29,887 |
38,427 |
17.11 |
02/28/2031 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2022 |
(4) |
--- |
--- |
--- |
--- |
49,754 |
163,193 |
--- |
--- |
--- |
|
03/01/2022 |
(5) |
21,583 |
93,524 |
10.21 |
02/28/2032 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2022 |
(6) |
--- |
--- |
--- |
--- |
--- |
--- |
26,160 |
10.21 |
02/28/2032 |
Amar |
04/20/2020 |
(7) |
--- |
--- |
--- |
--- |
20,628 |
67,660 |
--- |
--- |
--- |
Murugan |
04/20/2020 |
(3) |
73,333 |
36,667 |
8.20 |
04/19/2030 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2021 |
(4) |
--- |
--- |
--- |
--- |
23,493 |
77,057 |
--- |
--- |
--- |
|
03/01/2021 |
(5) |
27,575 |
35,453 |
17.11 |
02/28/2031 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2022 |
(4) |
--- |
--- |
--- |
--- |
42,100 |
138,088 |
--- |
--- |
--- |
|
03/01/2022 |
(5) |
18,262 |
79,136 |
10.21 |
02/28/2032 |
--- |
--- |
--- |
--- |
--- |
|
03/01/2022 |
(6) |
--- |
--- |
--- |
--- |
--- |
--- |
22,136 |
10.21 |
02/28/2032 |
51
2022 Option Exercises and Stock Vested
The following table sets forth certain information concerning the option awards exercised and stock awards vested for our named executive officers in 2022.
|
Option Awards |
Stock Awards |
||
Name |
Number of shares acquired |
Value Realized on |
Number of Shares |
Value Realized on Vesting ($)(2) |
Pascal Touchon, D.V.M. |
--- |
--- |
128,776 |
814,040 |
Charlene Banard |
--- |
--- |
--- |
--- |
Jakob Dupont, M.D. |
10,000 |
64,250 |
39,746 |
238,585 |
Utpal Koppikar |
--- |
--- |
55,025 |
367,207 |
Amar Murugan |
--- |
--- |
33,898 |
207,024 |
Pension Benefits
We do not have a defined benefit plan. Our named executive officers did not participate in, or otherwise receive, any special benefits under a defined benefit retirement plan sponsored by us during 2022.
Nonqualified Deferred Compensation
During 2022, our named executive officers did not contribute to, or earn any amount with respect to, any defined contribution or other plan sponsored by us that provides for the deferral of compensation on a basis that is not tax-qualified.
Employment Contracts and Change in Control Arrangements
Pascal Touchon, D.V.M.
We entered into an executive employment agreement with Dr. Touchon in May 2019 (the “Touchon Employment Agreement”) in connection with him joining the Company as our President and Chief Executive Officer in June 2019.
52
Under the Touchon Employment Agreement and the agreements governing his equity awards, he is entitled to certain benefits in the event of a termination of employment without cause or resignation for good reason. In the event Dr. Touchon’s employment is terminated by us without cause or he resigns for good reason, in either case, unrelated to a change in control (other than as a result of his death or disability), he will be entitled to receive the following benefits:
In addition, in the event Dr. Touchon’s employment is terminated by us without cause (other than as a result of his death or disability) at any time beginning three months before a change in control and ending twelve months following a change in control (the “change in control period”), or he resigns for good reason at any time during the change in control period, he will be entitled to receive the following payments and benefits:
In the event that Dr. Touchon’s employment is terminated by us as a result of his death or disability, he (or his heirs or estate) will be entitled to receive the following payments and benefits:
The receipt of any termination-based payments or benefits by Dr. Touchon is subject to his execution and the effectiveness of a release of claims against the Company.
Pursuant to the Touchon Employment Agreement, if any payments or benefits provided to Dr. Touchon in connection with a change in control are subject to excise taxes as a result of the application of Sections 280G and 4999 of the Internal Revenue Code, such payments and benefits will be reduced so that no excise tax is payable, but only if this reduction results in a more favorable after-tax position for him.
53
Charlene Banard
We entered into an executive employment agreement with Ms. Banard in March 2022 (“Banard Employment Agreement”) in connection with her joining the Company as our Executive Vice President, Chief Technical Officer in April 2022.
Under the Banard Employment Agreement and the agreements governing her equity awards, she is entitled to certain benefits in the event of a termination of employment without cause or resignation for good reason. In the event Ms. Banard’s employment is terminated by us without cause or she resigns for good reason, in either case, unrelated to a change in control (other than as a result of her death or disability), she will be entitled to receive the following benefits:
In addition, in the event Ms. Banard’s employment is terminated by us without cause (other than as a result of her death or disability) or as a result of a resignation for good reason, in either case, during the change in control period, she will be entitled to receive the following payments and benefits:
The receipt of any termination-based payments or benefits by Ms. Banard is subject to her execution and the effectiveness of a release of claims against the Company.
Pursuant to the Banard Employment Agreement, if any payments or benefits provided to Ms. Banard in connection with a change in control are subject to excise taxes as a result of the application of Sections 280G and 4999 of the Internal Revenue Code, such payments and benefits will be reduced so that no excise tax is payable, but only if this reduction results in a more favorable after-tax position for her.
Jakob Dupont, M.D.
We entered into an executive employment agreement with Dr. Dupont in May 2020 (“Dupont Employment Agreement”) in connection with his joining the Company as our Executive Vice President, Head of Research and Development in May 2020.
Under the Dupont Employment Agreement and the agreements governing his equity awards, he is entitled to certain benefits in the event of a termination of employment without cause or resignation for good reason. In the event Dr. Dupont’s employment is terminated by us without cause or he resigns for good reason, in either case, unrelated to a change in control (other than as a result of his death or disability), he will be entitled to receive the following benefits:
54
In addition, in the event Dr. Dupont’s employment is terminated by us without cause (other than as a result of his death or disability) or as a result of a resignation for good reason, in either case, during the change in control period, he will be entitled to receive the following payments and benefits:
The receipt of any termination-based payments or benefits by Dr. Dupont is subject to his execution and the effectiveness of a release of claims against the Company.
Pursuant to the Dupont Employment Agreement, if any payments or benefits provided to Dr. Dupont in connection with a change in control are subject to excise taxes as a result of the application of Sections 280G and 4999 of the Internal Revenue Code, such payments and benefits will be reduced so that no excise tax is payable, but only if this reduction results in a more favorable after-tax position for him.
Utpal Koppikar
We entered into an executive employment agreement with Mr. Koppikar in May 2018 in connection with his joining the Company in June 2018 as our Senior Vice President, Chief Financial Officer. In November 2020, we amended and restated Mr. Koppikar’s executive employment agreement in a form consistent with our form of executive employment agreement previously filed with the SEC (the “Amended Koppikar Employment Agreement”). Mr. Koppikar was promoted to Executive Vice President, Chief Financial Officer in February 2022. Mr. Koppikar notified the Company of his intention to resign as the Company’s Executive Vice President, Chief Financial Officer, effective March 31, 2023. Mr. Koppikar did not receive any severance benefits in connection with his resignation.
Under the Amended Koppikar Employment Agreement and the agreements governing his equity awards, he is entitled to certain benefits in the event of a termination of employment without cause or resignation for good reason. In the event Mr. Koppikar’s employment is terminated by us without cause or he resigns for good reason, in either case, unrelated to a change in control (other than as a result of his death or disability), he will be entitled to receive the following benefits:
55
In addition, in the event Mr. Koppikar’s employment is terminated by us without cause (other than as a result of his death or disability) or as a result of a resignation for good reason, in either case, during the change in control period, he will be entitled to receive the following payments and benefits:
The receipt of any termination-based payments or benefits by Mr. Koppikar is subject to his execution and the effectiveness of a release of claims against the Company.
Pursuant to the Amended Koppikar Employment Agreement, if any payments or benefits provided to Mr. Koppikar in connection with a change in control are subject to excise taxes as a result of the application of Sections 280G and 4999 of the Internal Revenue Code, such payments and benefits will be reduced so that no excise tax is payable, but only if this reduction results in a more favorable after-tax position for him.
Amar Murugan
We entered into an executive employment agreement with Mr. Murugan in April 2020 (the “Murugan Employment Agreement”) in connection with his joining the Company as our Senior Vice President, General Counsel. Mr. Murugan was promoted to Executive Vice President, Chief Legal Officer in March 2023.
Under the Murugan Employment Agreement and the agreements governing his equity awards, he is entitled to certain benefits in the event of a termination of employment without cause or resignation for good reason. In the event Mr. Murugan’s employment is terminated by us without cause or he resigns for good reason, in either case, unrelated to a change in control (other than as a result of his death or disability), he will be entitled to receive the following benefits:
56
In addition, in the event Mr. Murugan’s employment is terminated by us without cause (other than as a result of his death or disability) or as a result of a resignation for good reason, in either case, during the change in control period, he will be entitled to receive the following payments and benefits:
The receipt of any termination-based payments or benefits by Mr. Murugan is subject to his execution and the effectiveness of a release of claims against the Company.
Pursuant the Murugan Employment Agreement, if any payments or benefits provided to Mr. Murugan in connection with a change in control are subject to excise taxes as a result of the application of Sections 280G and 4999 of the Internal Revenue Code, such payments and benefits will be reduced so that no excise tax is payable, but only if this reduction results in a more favorable after-tax position for him.
57
Potential Payments Upon Termination or Change of Control
The amount of compensation and benefits payable to each named executive officer in various termination and change in control situations has been estimated in the tables below. The value of the option and RSU vesting accelerations was calculated for each of the tables below on the assumption that the change in control and executive’s employment termination occurred on December 31, 2022. The closing price of our common stock on December 31, 2022 was $3.28, which was used as the value of our common stock in the change in control calculations. The value of the option vesting acceleration was calculated by multiplying the number of unvested option shares subject to vesting acceleration as of December 31, 2022 by the difference between the closing price of our common stock as of December 31, 2022 and the exercise price. No value is attributed to unvested options subject to acceleration which have exercise prices above the closing market price of our common stock on December 31, 2022. The value of RSUs was calculated by multiplying the number of unvested RSUs subject to vesting acceleration as of December 31, 2022 by the closing price of our common stock on December 31, 2022.
Name |
Termination by |
Termination by |
Termination by |
Termination by |
Pascal Touchon, D.V.M. |
|
|
|
|
Base salary continuation |
723,450 |
1,446,900 |
--- |
--- |
Bonus continuation |
--- |
940,486 |
--- |
--- |
Lump sum bonus payment |
470,243 |
--- |
470,243 |
--- |
COBRA premiums |
20,289 |
40,578 |
--- |
--- |
Accelerated vesting of equity awards |
459,400 |
943,423 |
--- |
--- |
|
|
|
|
|
Charlene Banard |
|
|
|
|
Base salary continuation |
430,000 |
430,000 |
--- |
--- |
Lump sum bonus payment |
--- |
193,500 |
--- |
--- |
COBRA premiums |
20,289 |
20,289 |
--- |
--- |
Accelerated vesting of equity awards |
--- |
509,548 |
--- |
--- |
|
|
|
|
|
Jakob Dupont, M.D. |
|
|
|
|
Base salary continuation |
546,000 |
546,000 |
--- |
--- |
Lump sum bonus payment |
--- |
245,700 |
--- |
--- |
COBRA premiums |
29,278 |
29,278 |
--- |
--- |
Accelerated vesting of equity awards |
--- |
365,543 |
--- |
--- |
|
|
|
|
|
Utpal Koppikar |
|
|
|
|
Base salary continuation |
504,348 |
504,348 |
--- |
--- |
Lump sum bonus payment |
--- |
226,956 |
--- |
--- |
COBRA premiums |
--- |
--- |
--- |
--- |
Accelerated vesting of equity awards |
--- |
329,269 |
--- |
--- |
|
|
|
|
|
Amar Murugan |
|
|
|
|
Base salary continuation |
515,000 |
515,000 |
--- |
--- |
Lump sum bonus payment |
--- |
206,000 |
--- |
--- |
COBRA premiums |
29,278 |
29,278 |
--- |
--- |
Accelerated vesting of equity awards |
--- |
282,805 |
--- |
--- |
58
2022 Non-Employee Director Compensation
The following table sets forth information regarding compensation earned by or paid to our non-employee directors during 2022.
Name |
Fees Earned or |
Stock |
Option |
Total ($) |
Roy Baynes, M.D., Ph.D.(3) |
52,500 |
150,675 |
149,672 |
352,847 |
Eric L. Dobmeier |
70,000 |
150,675 |
149,672 |
370,347 |
Matthew K. Fust |
70,000 |
150,675 |
149,672 |
370,347 |
Carol Gallagher, Pharm.D. |
57,500 |
150,675 |
149,672 |
357,847 |
William K. Heiden |
55,000 |
150,675 |
149,672 |
355,347 |
Ameet Mallik |
45,000 |
150,675 |
149,672 |
345,347 |
Ronald C. Renaud, Jr.(4) |
90,000 |
150,675 |
149,672 |
390,347 |
Maria Grazia Roncarolo, M.D. |
45,000 |
150,675 |
149,672 |
345,347 |
Beth Seidenberg, M.D. |
55,000 |
150,675 |
149,672 |
355,347 |
|
|
|
|
|
Our non-employee director compensation policy, pursuant to which we compensate our non-employee directors with a combination of cash and equity, is intended to be fair and competitive to account for the time and effort required of a director of the Company. The annual cash compensation contained in this policy, set forth below, is payable in equal quarterly installments, in arrears following the end of each quarter in which the service occurred, pro-rated for any partial months of service. The Compensation Committee reviews pay levels for non-employee directors regularly with assistance from its compensation consultant, who prepares a comprehensive assessment of our non-employee director compensation program. That assessment includes benchmarking of director compensation against the same peer group used for executive compensation purposes and an update on recent trends in director compensation. Following that review, the Compensation Committee approved changes to the equity compensation in the non-employee director compensation policy for 2022. Our director compensation is set forth below:
All Directors other than Lead Director or Chair of the Board: $45,000
Lead Director: $75,000
Chair of the Board: $85,000
Chair of the Audit Committee: $20,000
Chair of the Compensation Committee: $15,000
Chair of the Nominating and Corporate Governance Committee: $10,000
59
Audit Committee: $10,000
Compensation Committee: $7,500
Nominating and Corporate Governance Committee: $5,000
Research and Development Committee: $5,000
We have reimbursed, and under this policy will continue to reimburse, our non-employee directors for their travel, lodging and other reasonable expenses incurred in attending meetings of our Board and committees of our Board.
The non-employee director compensation policy also provides for equity compensation to each non-employee director as follows:
All options granted to our non-employee directors under the policy will vest in full upon the completion of a change in control. Grant date option value is determined using the same method we use to calculate the grant date fair value of stock options in our financial statements.
Equity Compensation Plan Information
The following table provides certain information with respect to all of our equity compensation plans in effect as of December 31, 2022.
Plan Category |
Number of Securities to be Issued |
Weighted-Average Exercise |
Number of Securities |
Equity Compensation Plans Approved by Stockholders(2) |
14,078,975 |
$17.78 |
5,378,142 |
Equity Compensation Plans Not Approved by Stockholders(3) |
3,275,188 |
$13.11 |
1,916,728 |
Total |
17,354,163 |
$16.88 |
7,294,870 |
60
Pay Versus Performance
The following table sets forth information regarding the Company’s performance and the “compensation actually paid” to our named executive officers, as calculated in accordance with SEC disclosure rules:
|
|
|
|
|
|
|
|
|
|
Value of Initial Fixed $100 Investment Based on:(4) |
|
|
|
|
||
Year(1) |
|
Summary Compensation Table Total for PEO(2) ($) |
|
Compensation Actually Paid to PEO(3) ($) |
|
Average Summary Compensation Table Total for Non-PEO Named Executive Officers(2) ($) |
|
Average Compensation Actually Paid to Non-PEO Named Executive Officers(3) ($) |
|
Total Shareholder Return ($) |
|
Peer Group Total Shareholder Return(5) ($) |
|
Net Income (Loss) ($) |
|
Company Selected Measure(6) |
2022 |
|
|
( |
|
|
( |
|
|
|
|
N/A |
|||||
2021 |
|
|
|
|
|
|
|
( |
|
N/A |
||||||
2020 |
|
|
|
|
|
|
|
( |
|
N/A |
||||||
- 2022: Jakob Dupont, M.D.; Utpal Koppikar; Amar Murugan; and Charlene Banard
- 2021: Jakob Dupont, M.D.; Utpal Koppikar; Amar Murugan; and Kristin Yarema, Ph.D.
- 2020: Jakob Dupont, M.D.; Utpal Koppikar; Joseph Newell; and Kristin Yarema, Ph.D.
61
Compensation Actually Paid Adjustments
Year |
|
Summary Compensation Table Total ($)(1) |
|
(Minus) Grant Date Fair Value of Stock Option and Stock Awards Granted in Fiscal Year ($)(2) |
|
Plus Fair Value at Fiscal Year-End of Outstanding and Unvested Stock Option and Stock Awards Granted in Fiscal Year ($)(3) |
|
Plus/(Minus) Change in Fair Value of Outstanding and Unvested Stock Option and Stock Awards Granted in Prior Fiscal Years ($)(4) |
|
Plus Fair Value at Vesting of Stock Option and Stock Awards Granted in Fiscal Year that Vested During Fiscal Year ($)(5) |
|
Plus/(Minus) Change in Fair Value as of Vesting Date of Stock Option and Stock Awards Granted in Prior Fiscal Years for which Applicable Vesting Conditions Were Satisfied During Fiscal Year ($)(6) |
|
(Minus) Fair Value as of Prior Fiscal Year-End of Stock Option and Stock Awards Granted in Prior Fiscal Years that Failed to Meet Applicable Vesting Conditions During Fiscal Year ($)(7) |
|
Equals Compensation Actually Paid ($) |
Pascal Touchon, D.V.M. |
||||||||||||||||
2022 |
|
|
( |
|
|
( |
|
|
( |
|
|
( |
||||
2021 |
|
|
( |
|
|
( |
|
|
( |
|
|
|||||
2020 |
|
|
( |
|
|
|
|
( |
|
|
||||||
Other Named Executive Officers (Average) (8) |
||||||||||||||||
2022 |
|
|
( |
|
|
( |
|
|
( |
|
|
( |
||||
2021 |
|
|
( |
|
|
( |
|
|
( |
|
|
|||||
2020 |
|
|
( |
|
|
|
|
( |
|
|
||||||
Relationship Between Pay and Performance
We believe the “compensation actually paid” or “CAP” in each of the years reported above and over the three-year cumulative period are reflective of the Compensation Committee’s emphasis on “pay-for-performance" as the “compensation actually paid” fluctuated year-over-year, primarily due to the result of our stock performance and our varying levels of achievement against pre-established performance goals under our annual incentive program.
As noted above, as is the case with many companies in the biotechnology industry, the Company’s incentive objectives are generally tied to our strategic and operational goals rather than financial goals. Accordingly, our compensation program is not influenced by financial metrics, such as net income. For 2020, our net loss was $306,620,000 as compared to the “compensation actually paid” of $7,134,152 for Dr. Touchon and $3,649,489 for the average of our other named executive officers. In 2021, our net loss was 340,141,000 while the “compensation actually paid” paid for Dr. Touchon and for other named executive officers decreased to $428,098 and $1,138,071, respectively. With respect to 2022, our net loss was $228,302,000, while the “compensation actually paid” decreased to $(2,901,098) for Dr. Touchon and $(281,950) for our other named executive officers. The fluctuations in our “compensation actually paid” were driven by the fluctuations in our stock price over the
62
three-year period, particularly in light of the leverage of our executive compensation program towards equity awards. The following graphic illustrates the relationship between the “compensation actually paid” to the named executive officers and the Company’s total shareholder return (“TSR”) as well as the relationship between the Company’s TSR and the TSR of the Nasdaq Biotechnology Index, an independently prepared index that includes companies in the biotechnology industry.
Description of Relationship Between PEO and Average NEO Compensation Actually Paid and Our TSR
The following chart sets forth the relationship between CAP to our PEO, the average of CAP to our other NEOs, each as set forth in the table above, and our TSR over the three-year period from 2020 through 2022.
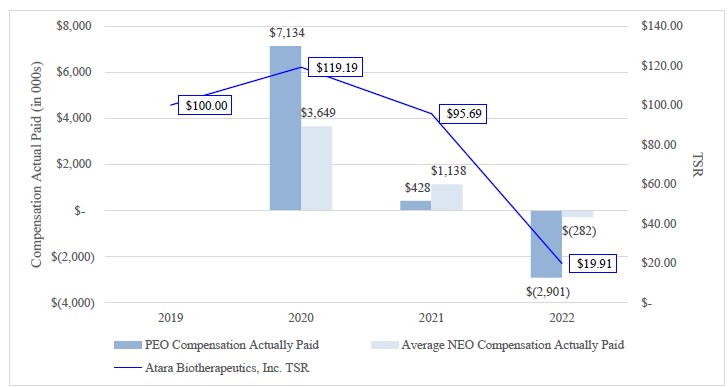
63
Description of Relationship Between Our TSR and Peer Group Index TSR
The following chart compares our TSR over the three-year period from 2020 through 2022 to that of the NASDAQ Biotechnology Index over the same time period.
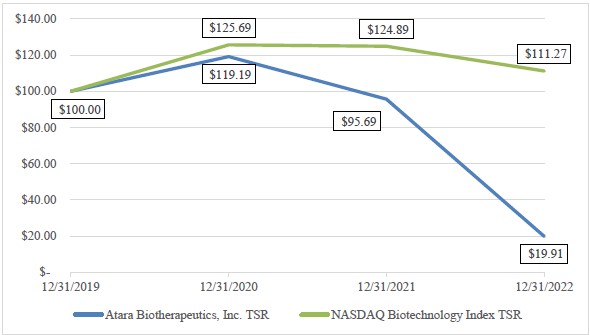
Performance Measures Used to Link Company Performance and Compensation Actually Paid to the NEOs
As noted in the CD&A, for 2022, the principal incentive elements in our executive compensation program were delivered in the form of annual incentives and equity awards. As is the case with many companies in the biotechnology industry, our annual incentive objectives are generally tied to the Company’s strategic and operational goals rather than financial goals. The following is a list of performance measures, which in our assessment represent the most important performance measures used by the Company to link compensation actually paid to the named executive officers for 2022:
64
65
Policies and Procedures for Transactions with Related Persons
We have adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock, any members of the immediate family of any of the foregoing persons and any firm, corporation or other entity in which any of the foregoing persons is an executive partner or principal or which such person has a 5% or greater beneficial ownership interest (each a “Related Person”), are not permitted to enter into a Related Person transaction with us without the prior consent of the Audit Committee. Any request for us to enter into a transaction with a Related Person, in which the amount involved exceeds $120,000 and such person would have a direct or indirect interest, must first be presented to the Audit Committee for review, consideration and approval. In approving or rejecting any such proposal, the Audit Committee is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the Related Person’s interest in the transaction.
Certain Related Person Transactions
Since January 1, 2023, we have not entered into any transactions, nor are any such transactions currently proposed, to which we were a party and in which the amount involved exceeded $120,000, and in which Related Person had or will have a direct or indirect material interest, other than compensation arrangements which are described under “Executive Compensation” and “Non-Employee Director Compensation.”
Indemnification Agreements
We have entered into indemnity agreements with our directors and officers that provide, among other things, that we will indemnify such officer or director, under the circumstances and to the extent provided for therein, for all reasonable expenses and liabilities incurred with any action or proceeding brought against them by reason of the fact that they are serving in such capacity, and otherwise to the fullest extent permitted under Delaware law and our Bylaws.
Merck Relationship
In April 2017, we entered into an agreement with Merck Sharp & Dohme (known as MSD outside of the U.S. and Canada) to provide drug supply for a study to be sponsored and conducted by us to evaluate our tab-cel® product candidate in combination with Merck’s anti-PD-1 (programmed death receptor-1) therapy, KEYTRUDA® (pembrolizumab), in patients with platinum-resistant or recurrent Epstein-Barr virus-associated nasopharyngeal carcinoma. The Phase 1/2 study achieved its safety endpoints and stable disease in some patients. We are currently finalizing the clinical study report for this study. Dr. Baynes, who was a member of our Board from September 2018 to December 2022, was the Senior Vice President Global Clinical Development and Chief Medical Officer at Merck & Co., Inc., which is an affiliate of Merck Sharp & Dohme until July 2022.
66
HOUSEHOLDING OF PROXY MATERIALS
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for Notices of Internet Availability of Proxy Materials or other Annual Meeting materials with respect to two or more stockholders sharing the same address by delivering a single Notice of Internet Availability of Proxy Materials or other Annual Meeting materials addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
A number of brokers with account holders who are Atara stockholders will be “householding” our proxy materials. A single Notice of Internet Availability of Proxy Materials will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate Notice of Internet Availability of Proxy Materials, please notify your broker or Atara. Direct your written request to Atara Biotherapeutics, Inc., Investor Relations, 2380 Conejo Spectrum Street, Suite 200, Thousand Oaks, CA 91320 or contact Investor Relations at 805-395-9669. In addition, Atara will promptly deliver, upon written or oral request to the address or telephone number above, a separate copy of the Notice of Internet Availability of Proxy Materials or the full set of proxy materials, as applicable, to a stockholder at a shared address to which a single copy of the documents was delivered. Stockholders who currently receive multiple copies of the Notices of Internet Availability of Proxy Materials at their addresses and would like to request “householding” of their communications should contact their brokers.
OTHER MATTERS
The Board knows of no other matters that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before the Annual Meeting, it is the intention of the persons named in the accompanying proxy to vote on such matters in accordance with their best judgment.
By Order of the Board of Directors
/s/ Pascal Touchon
Pascal Touchon, D.V.M.
President and Chief Executive Officer
April [__], 2023
A copy of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 is available without charge upon written request to: Secretary, Atara Biotherapeutics, Inc., 2380 Conejo Spectrum Street, Suite 200, Thousand Oaks, CA 91320.
67
EXHIBIT A
SECOND AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
ATARA BIOTHERAPEUTICS, INC.
Pascal Touchon hereby certifies that:
ONE: The original name of this corporation is Atara, Inc. and the date of filing the original Certificate of Incorporation of this corporation with the Secretary of State of the State of Delaware was August 22, 2012 and was amended and restated on October 21, 2014.
TWO: He is the duly elected and acting President and Chief Executive Officer of Atara Biotherapeutics, Inc., a Delaware corporation.
THREE: The Certificate of Incorporation of this corporation is hereby further amended and restated to read as follows:
I.
The name of this corporation is Atara Biotherapeutics, Inc. (the “Company”).
II.
The address of the registered office of the Company in the State of Delaware is 251 Little Falls Drive, City of Wilmington, County of New Castle, 19808 and the name of the registered agent of the Company in the State of Delaware at such address is Corporation Service Company.
III.
The purpose of the Company is to engage in any lawful act or activity for which a corporation may be organized under the Delaware General Corporation Law (“DGCL”).
IV.
A. The Company is authorized to issue two classes of stock to be designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares that the Company is authorized to issue is 520,000,000 shares. 500,000,000 shares shall be Common Stock, each having a par value of $0.0001. 20,000,000 shares shall be Preferred Stock, each having a par value of $0.001.
B. The Preferred Stock may be issued from time to time in one or more series. The Board of Directors is hereby expressly authorized to provide for the issue of all of any of the shares of the Preferred Stock in one or more series, and to fix the number of shares and to determine or alter for each such series, such voting powers, full or limited, or no voting powers, and such designation, preferences, and relative, participating, optional, or other rights and such qualifications, limitations, or restrictions thereof, as shall be stated and expressed in the resolution or resolutions adopted by the Board of Directors providing for the issuance of such shares and as may be permitted by the DGCL. The Board of Directors is also expressly authorized to increase or decrease the number of shares of any series subsequent to the issuance of shares of that series, but not below the number of shares of such series then outstanding. In case the number of shares of any series shall be decreased in accordance with the foregoing sentence, the shares constituting such decrease shall resume the status that they had prior to the adoption of the resolution originally fixing the number of shares of such series.
C. The number of authorized shares of Preferred Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of the stock of the Company entitled to vote thereon, without a separate vote of the holders of the Preferred Stock, or of any series thereof, unless a vote of any such holders is required pursuant to the terms of any certificate of designation filed with respect to any series of Preferred Stock.
68
D. Each outstanding share of Common Stock shall entitle the holder thereof to one vote on each matter properly submitted to the stockholders of the Company for their vote; provided, however, that, except as otherwise required by law, holders of Common Stock shall not be entitled to vote on any amendment to this Certificate of Incorporation (including any certificate of designation filed with respect to any series of Preferred Stock) that relates solely to the terms of one or more outstanding series of Preferred Stock if the holders of such affected series are entitled, either separately or together as a class with the holders of one or more other such series, to vote thereon by law or pursuant to this Certificate of Incorporation (including any certificate of designation filed with respect to any series of Preferred Stock).
V.
For the management of the business and for the conduct of the affairs of the Company, and in further definition, limitation and regulation of the powers of the Company, of its directors and of its stockholders or any class thereof, as the case may be, it is further provided that:
A. Board of Directors.
1. Generally. The management of the business and the conduct of the affairs of the Company shall be vested in its Board of Directors. The number of directors that shall constitute the Board of Directors shall be fixed exclusively by resolutions adopted by a majority of the authorized number of directors constituting the Board of Directors.
2. Board of Directors. Subject to the rights of the holders of any series of Preferred Stock to elect additional directors under specified circumstances, the directors shall be divided into three classes designated as Class I, Class II and Class III, respectively. The Board of Directors is authorized to assign members of the Board of Directors already in office to such classes at the time the classification becomes effective. At the first annual meeting of stockholders following the initial classification, the term of office of the Class I directors shall expire and Class I directors shall be elected for a full term of three years. At the second annual meeting of stockholders following the initial classification, the term of office of the Class II directors shall expire and Class II directors shall be elected for a full term of three years. At the third annual meeting of stockholders following the initial classification, the term of office of the Class III directors shall expire and Class III directors shall be elected for a full term of three years. At each succeeding annual meeting of stockholders, directors shall be elected for a full term of three years to succeed the directors of the class whose terms expire at such annual meeting. Notwithstanding the foregoing provision of this section, each director shall serve until his or her successor is duly elected and qualified or until his or her earlier death, resignation or removal. No decrease in the number of directors constituting the Board of Directors shall shorten the term of any incumbent director.
3. Removal of Directors. Subject to the rights of any series of Preferred Stock to elect additional directors under specified circumstances, neither the Board of Directors nor any individual director may be removed without cause. Subject to any limitations imposed by applicable law, any individual director or directors may be removed without cause by the affirmative vote of the holders of 66 2/3% of the voting power of all then outstanding shares of capital stock of the Company entitled to vote generally at an election of directors.
4. Vacancies. Subject to any limitations imposed by applicable law and subject to the rights of the holders of any series of Preferred Stock, any vacancies on the Board of Directors resulting from death, resignation, disqualification, removal or other causes and any newly created directorships resulting from any increase in the number of directors, shall, unless the Board of Directors determines by resolution that any such vacancies or newly created directorships shall be filled by the stockholders and except as otherwise provided by applicable law, be filled only by the affirmative vote of a majority of the directors then in office, even though less than a quorum of the Board of Directors, and not by the stockholders. Any director elected in accordance with the preceding sentence shall hold office for the remainder of the full term of the director for which the vacancy was created or occurred and until such director’s successor shall have been elected and qualified.
B. Stockholder Actions. No action shall be taken by the stockholders of the Company except at an annual or special meeting of stockholders called in accordance with the Bylaws. Advance notice of stockholder nominations for the election of directors and of business to be brought by stockholders before any meeting of the stockholders of the Company shall be given in the manner provided in the Bylaws of the Company.
C. Bylaws. The Board of Directors is expressly empowered to adopt, amend or repeal the Bylaws of the Company. Any adoption, amendment or repeal of the Bylaws of the Company by the Board of Directors shall require the approval of a majority of the authorized number of directors. The stockholders shall also have power to adopt, amend or repeal the Bylaws of the Company; provided, however, that, in addition to any vote of the holders of any class or series of stock of the Company required
69
by law or by this Certificate of Incorporation, such action by stockholders shall require the affirmative vote of the holders of at least 66 2/3% of the voting power of all of the then-outstanding shares of the capital stock of the Company entitled to vote generally in the election of directors, voting together as a single class.
VI.
A. The personal liability of the directors or officers for monetary damages for the breach of fiduciary duty as a director or officer shall be eliminated to the fullest extent under applicable law, as it presently exists or may hereafter be amended from time to time.
B. To the fullest extent permitted by applicable law, the Company is authorized to provide indemnification of (and advancement of expenses to) directors, officers and agents of the Company (and any other persons to which applicable law permits the Company to provide indemnification) through Bylaw provisions, agreements with such agents or other persons, vote of stockholders or disinterested directors or otherwise in excess of the indemnification and advancement otherwise permitted by such applicable law. If applicable law is amended after approval by the stockholders of this Article VI to authorize corporate action further eliminating or limiting the personal liability of directors or officers, then the liability of a director or officer to the company shall be eliminated or limited to the fullest extent permitted by applicable law as so amended. For purposes of this Article VI, “officer” shall have the meaning provided in Section 102(b)(7) of the DGCL, as it presently exists or may hereafter be amended from time to time.
C. Any repeal or modification of this Article VI shall only be prospective and shall not affect the rights or protections or increase the personal liability of any director or officer under this Article VI in effect at the time of the alleged occurrence of any act or omission to act giving rise to liability or indemnification.
D. Unless the Company consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (i) any derivative action or proceeding brought on behalf of the Company; (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company to the Company or the Company’s stockholders; (iii) any action asserting a claim against the Company or any director or officer or other employee of the Company arising pursuant to any provision of the DGCL, the Amended and Restated Certificate of Incorporation or the Bylaws of the Company; or (iv) any action asserting a claim against the Company or any director or officer or other employee of the Company governed by the internal affairs doctrine.
VII.
A. The Company reserves the right to amend, alter, change or repeal any provision contained in this Certificate of Incorporation, in the manner now or hereafter prescribed by statute, except as provided in paragraph B. of this Article VII, and all rights conferred upon the stockholders herein are granted subject to this reservation.
B. Notwithstanding any other provisions of this Certificate of Incorporation or any provision of law that might otherwise permit a lesser vote or no vote, but in addition to any affirmative vote of the holders of any particular class or series of stock of the Company required by law or by this Certificate of Incorporation or any certificate of designation filed with respect to a series of Preferred Stock, the affirmative vote of the holders of at least 66 2/3% of the voting power of all of the then-outstanding shares of capital stock of the Company entitled to vote generally in the election of directors, voting together as a single class, shall be required to alter, amend or repeal Articles V, VI, and VII.
* * * *
FOUR: This Amended and Restated Certificate of Incorporation has been duly approved by the Board of Directors of this corporation.
FIVE: This Amended and Restated Certificate of Incorporation was approved by the holders of the requisite number of shares of this corporation in accordance with Section 228 of the DGCL. This Amended and Restated Certificate of Incorporation has been duly adopted in accordance with the provisions of Sections 242 and 245 of the DGCL by the stockholders of this corporation.
70
ATARA BIOTHERAPEUTICS, INC. has caused this Amended and Restated Certificate of Incorporation to be signed by its President and Chief Executive Officer on [•], 2023.
|
Atara Biotherapeutics, Inc. |
|
|
|
|
Pascal Touchon |
President and Chief Executive Officer |
71
ATARA BIOTHERAPEUTICS, INC. 2380 CONEIO SPECTRUM STREET, SUITE 200 THOUSAND OAKS, CA 91320 SCAN TO VIEW MATERIALS & VOTE VOTE BY INTERNET Before The Meeting – Go to www.proxyvote.com or scan the QR Barcode above Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 p.m. Eastern Time on May 29, 2023. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. During The Meeting – Go to www.virtualshareholdermeeting.com/ATRA2023 You may attend the meeting via the Internet and vote during the meeting. Have the information that is printed in the box marked by the arrow available and follow the instructions. VOTE BY PHONE – 1- 800-690-6903 Use any touch-tone telephone to transmit your voting instructions up until 11:59 p.m. Eastern Time on May 29, 2023. Have your proxy card in hand when you call and then follow the instructions. VOTE BY MAIL Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717. TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: VO1807-P89778 KEEP THIS PORTION FOR YOUR RECORDS DETACH AND RETURN THIS PORTION ONLY THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED. ATARA BIOTHERAPEUTICS,INC. The Board of Directors recommends you vote FOR the following: 1. Election of Directors Nominees: For Withhold 1a. Pascal Touchon, D.V.M. 1b. Carol Gallagher, Pharm.D. 1c. Maria Grazia Roncarolo, M.D. The Board of Directors recommends you vote FOR the following proposal: 2. To approve, on an advisory basis, the compensation of the Company's named executive officers, as disclosed in the Proxy Statement For Against Abstain The Board of Directors recommends you vote FOR the following proposal: For Against Abstain 3. To ratify the selection of Deloitte & Touche LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2023. The Board of Directors recommends you vote FOR the following proposal: For Against Abstain 4. To approve an amendment to the Company's Certificate of Incorporation to provide for the exculpation of officers as permitted by Delaware law. NOTE: Such other matters that may properly come before the meeting or any adjournment or postponement thereof will be voted on by the proxy holders in their discretion. Please sign exactly as your name(s) appear(s) hereon. When signing as attorney, executor, administrator, or other fiduciary, please give full title as such, Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or partnership name by authorized officer. Signature [PLEASE SIGN WITHINBOX] Date Signature (Joint Owners) Date

Important NoticeRegarding the Availability of Proxy Materials for the Annual Meeting: The Notice and Proxy Statement and 2022 AnnualReport on Form 10-K are available at www.proxyvote.com. V01808-P89778 ATARA BIOTHERAPEUTICS, INC. Annual Meeting of Stockholders May 30, 2023 9:00 AM PacificTime This proxy issolicited by the Board of Directors The undersigned stockholder herebyappoints Pascal Touchonand Eric Hyllengren, or either of them, as proxies, each with the power to appoint his substitute, and hereby authorizes them to represent and to vote, as designated on the reverse side of this ballot, all of the shares of common stock of ATARA BIOTHERAPEUTICS, INC. that thestockholder is entitled to vote at the Annual Meeting of Stockholders to be held at 9:00 AM Pacific Time on May 30, 2023 virtually at www.virtualshareholdermeeting.com/ATRA2023,and any adjournment or postponement thereof. This proxy, when properly executed, will be voted in the manner directedherein. If no such direction is made, this proxy will be voted FOR all nominees listed in Proposal 1 and FOR Proposals 2, 3 and 4 in accordance with the recommendations of the Board of Directors,and in the discretion of the proxy holders upon any other business as may properly come before the Annual Meeting of Stockholders or any adjournment or postponement thereof. Continued and to be signed on reverse side